Jun 27, 2025
Articles
Jun 27, 2025
Articles
Jun 27, 2025
Articles
Conformity Assessment US - Everything You Need To Know

Martin Ramirez

Martin Ramirez

Martin Ramirez

Did you know product recalls in the U.S. surged by 8% in the first quarter of 2024?
As safety concerns grow, regulators are tightening their grip on compliance through stricter conformity assessment practices.
In this guide, we’ll break down how conformity assessment works in the U.S., explore key regulatory frameworks, and share practical strategies to help your business stay compliant and competitive.
What is Conformity Assessment?
Conformity assessment refers to the processes used to evaluate whether products, services, systems, or personnel meet specific standards or requirements.
According to ISO/IEC 17000, it is the "demonstration that specified requirements relating to a product, process, system, person or body are fulfilled."
In the U.S. context, this includes testing, inspection, and certification activities that bridge the gap between standards and the marketplace, ensuring consistent safety, quality, and performance.
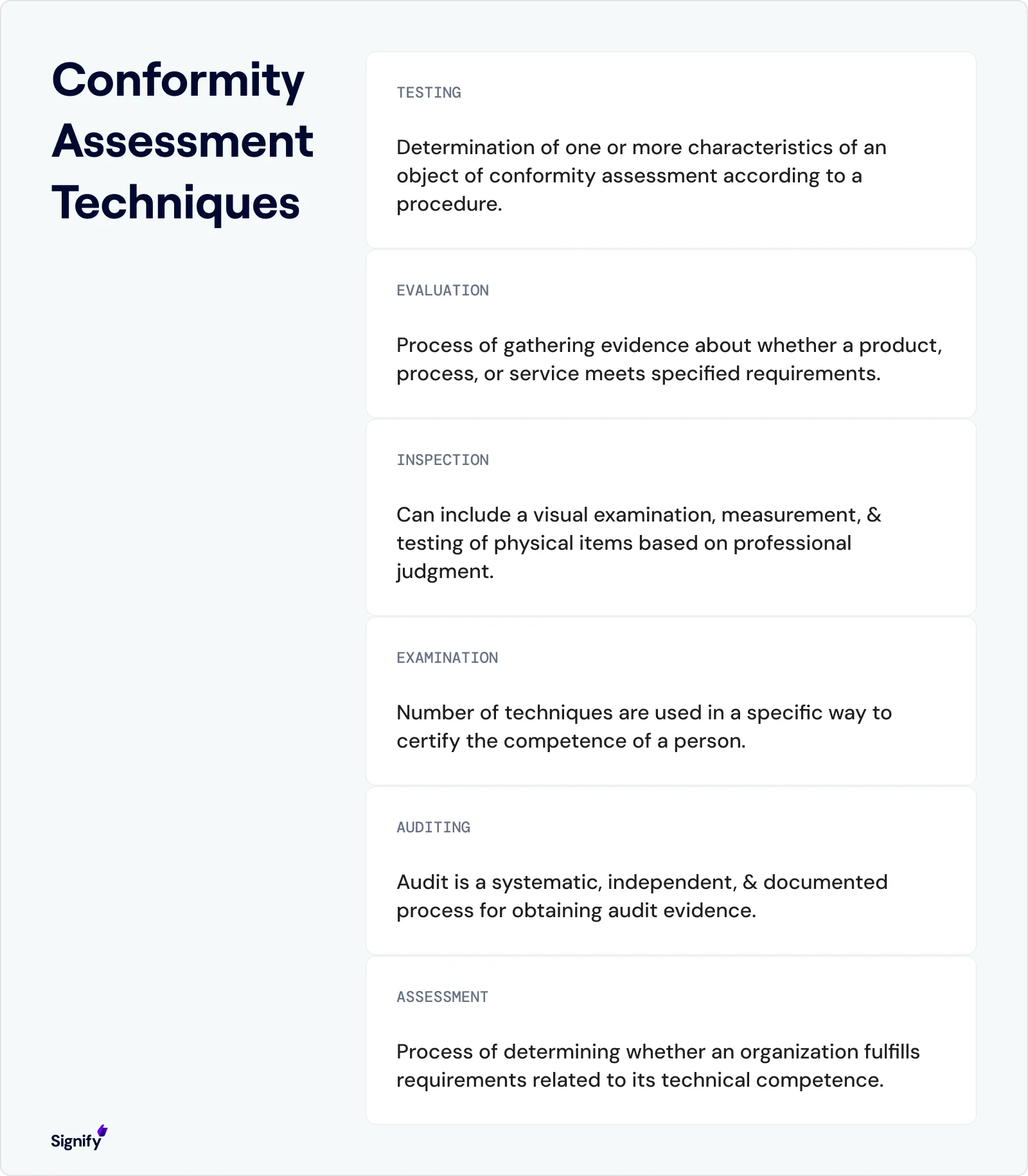
Why Focus on the U.S. Conformity Assessment System?
The U.S. system is decentralized and relies on collaboration between the public and private sectors.
Voluntary consensus standards play a major role, and government agencies often recognize or incorporate private-sector assessments.
Understanding how this hybrid system operates is crucial for ensuring compliance, facilitating market access, and fostering trust.
U.S. Standards and Regulatory Framework
1. Voluntary Consensus Standards & ANSI
The U.S. relies heavily on voluntary consensus standards (VCS) to support safety, interoperability, and compliance across industries.

Unlike countries with centralized standards bodies, the U.S. system is led by the private sector and decentralized, with coordination overseen by the American National Standards Institute (ANSI).
ANSI does not write standards itself. Instead, it:
Accredits standards developers,
Approves standards as American National Standards (ANS),
Ensures alignment with World Trade Organization Technical Barriers to Trade (WTO/TBT) principles.
➸ Key accredited developers include:
ASTM International: materials, construction, petroleum, environment
IEEE: electronics, telecommunications, power systems
ASME: boilers, piping, mechanical engineering
NFPA: fire protection and electrical safety
These organizations draft standards through open, consensus-driven processes, which are critical to product design, performance, and market access.
2. U.S. Government Regulatory Policies (NTTAA and OMB A-119)
To strengthen collaboration between the private sector and government, the U.S. adopted a policy framework that encourages federal reliance on voluntary standards and private-sector conformity assessment systems.
National Technology Transfer and Advancement Act (NTTAA)
Passed in 1995, the NTTAA (15 U.S.C. § 272) directs federal agencies to:
Use voluntary consensus standards (VCS) instead of government-unique standards whenever practical.
Participate in standard development activities to reflect public interests.
Report annually to the National Institute of Standards and Technology (NIST) on how VCSs are used.

➸ Example: Instead of writing a unique flame resistance test, a federal agency may adopt ASTM or NFPA fire standards.
This ensures that regulations are technology-neutral, globally aligned, and business-friendly.
OMB Circular A-119
Issued by the Office of Management and Budget (OMB), Circular A-119 provides specific guidance to federal agencies on implementing the NTTAA.

It instructs agencies to:
Prefer private-sector conformity assessment results when regulating or procuring products.
Avoid duplicating testing or certification processes that already exist in the market.
Engage early in standards development to shape outcomes aligned with regulatory needs.
➸ Impact: A product already certified by an ANSI-accredited body may not need retesting for federal procurement or compliance, saving businesses time and cost.
Together, the NTTAA and OMB A-119 promote regulatory efficiency, reduce technical barriers to trade, and foster innovation by integrating private sector expertise into federal processes.
Federal Guidance: 15 CFR Part 287
The Code of Federal Regulations Title 15, Part 287 provides overarching federal policy on how agencies should approach conformity assessment.
Issued by the National Institute of Standards and Technology (NIST), this regulation reinforces alignment with the NTTAA and OMB A-119, while also providing clarity on implementation.
Key Federal Objectives Under 15 CFR Part 287
NIST encourages agencies to adopt a coordinated, transparent, and risk-based approach to conformity assessment that serves the interests of both the public and private sectors.
➸ Agencies are guided to:
Minimize unnecessary regulatory burden on businesses.
Recognize and use accredited private-sector conformity assessment programs wherever appropriate.
Tailor conformity assessment activities based on risk, cost, and confidence level needed.
Promote transparency in the selection and application of assessments.
➸ Example: A low-risk product may only need a supplier’s declaration of conformity, while a high-risk product (like a medical device) may require third-party certification.
Federal-Private Sector Collaboration
15 CFR Part 287 also calls for:
Active collaboration with industry groups, trade associations, and standards bodies.
Efficient use of existing infrastructure, such as accredited labs and certification bodies.
Ongoing assessment of conformity practices to adapt to evolving technologies and risks.
➸ Impact: This framework reduces redundancy, enhances market confidence, and helps U.S. businesses meet both domestic and international requirements more efficiently.
U.S. National Conformity Assessment Principles (NCAP)
The U.S. National Conformity Assessment Principles (NCAP), developed by NIST, outline how conformity assessment should be applied across both public and private sectors.
These principles serve as a foundational policy framework to promote trust, reduce trade barriers, and ensure consistent application of conformity practices across industries.
Core Principles and WTO/TBT Alignment
NCAP aligns with the World Trade Organization (WTO) Agreement on Technical Barriers to Trade (TBT).
This alignment ensures that U.S. conformity practices support international trade while maintaining national regulatory objectives.

➸ Key principles include:
Avoiding unnecessary obstacles to trade.
Recognizing international equivalence of standards and assessments.
Treating domestic and foreign suppliers equally.
➸ Impact: A conformity certificate issued by an accredited lab in Europe may be accepted in the U.S. if it meets equivalent technical and accreditation criteria, reducing duplicative testing.
Purpose and Stakeholder Confidence
At its core, NCAP emphasizes that conformity assessment should deliver reliable, reproducible, and unbiased results, whether used by regulators, businesses, or consumers.
Confidence in results supports the enforcement of safety regulations, procurement decisions, and consumer trust.
Competency and impartiality of assessment bodies are essential for credibility.
➸ Example: When a testing lab is ISO/IEC 17025-accredited and follows NCAP principles, its results are more likely to be accepted across industries and borders.
Applicability Across Parties and Activities
NCAP is intentionally broad in scope. It applies not only to federal agencies but also to:
Private-sector certification bodies.
Inspection and testing labs.
Accreditation organizations.
Industry associations and trade groups.
The principles cover a wide range of conformity assessment activities, including:
Testing,
Inspection,
Certification,
Surveillance,
Accreditation.
➸ Benefit: This consistency ensures that different players in the conformity ecosystem operate with shared expectations and aligned procedures, reducing confusion and overlap.
Types of Conformity Assessment
Conformity assessment can be categorized into three levels of assurance based on the entity that performs the assessment.

Each type offers a different level of credibility, cost, and regulatory weight.
1. First-Party Assessment (Supplier’s Declaration of Conformity)
A first-party assessment is performed by the manufacturer or supplier themselves.
It’s often used in low-risk scenarios where internal controls are considered sufficient.
Involves self-testing, documentation, and a declaration that the product meets relevant standards.
Cost-effective and fast, but it relies entirely on internal processes and technical competency.
Common in industries where rapid innovation and short product cycles are prioritized.
➸ Example: A computer accessory manufacturer may self-declare compliance with FCC electromagnetic compatibility (EMC) requirements.
2. Second-Party Assessment (Customer-Driven Review)
A second-party assessment is conducted by the purchaser or client, usually a business vetting its suppliers or subcontractors.
Often includes on-site audits, documentation reviews, or product inspections.
Enables buyers to ensure their vendors meet quality, safety, or sustainability criteria.
Common in supply chain management and procurement settings.
➸ Example: A large retailer auditing its food suppliers for compliance with internal safety and labeling requirements.
3. Third-Party Assessment (Independent Certification)
A third-party assessment is conducted by a neutral, accredited organization with no stake in the outcome.
It is the most credible form of conformity assessment and is often required by law or industry regulations.
Provides objective, traceable, and standardized verification.
Recognized by regulators, buyers, and international markets.
Especially important for high-risk sectors, such as medical devices, electrical products, or construction materials.
➸ Example: A medical device certified by an FDA-accredited third-party review body or a consumer appliance certified by UL or Intertek for electrical safety.
Implementing U.S. Conformity Assessment
Conformity assessment in the U.S. follows a flexible, risk-based framework that aligns public oversight with private-sector expertise.
Agencies are encouraged to tailor their approaches based on risk, rely on accredited third-party bodies, and avoid redundant government processes.
Risk-Based Approach & Confidence/Regulation Spectrum
Federal policy encourages agencies to apply graduated levels of assessment depending on the product’s risk profile and the level of confidence required by regulators or consumers.
Assessment methods range from:
First-party declarations for low-risk, non-critical products.
Third-party certification for high-risk or safety-sensitive products.
Government-led review in highly regulated or national security sectors.
➸ Example: Toys may be self-declared for labeling accuracy, but children’s products with lead exposure risks require third-party testing under CPSC rules.
This model enables the U.S. system to strike a balance between oversight and innovation without imposing unnecessary regulatory burdens.
Federal Agency Use & Public/Private Alignment
Many federal agencies recognize or contract with private conformity assessment bodies instead of conducting assessments in-house.
This minimizes taxpayer costs while expanding access to qualified assessors.
➸ Example: The Occupational Safety and Health Administration (OSHA) recognizes Nationally Recognized Testing Laboratories (NRTLs), such as UL, to test electrical equipment, rather than operating its labs.
Other agencies, such as the EPA, FDA, and DOT, have similar frameworks that rely on private sector programs to meet their regulatory needs.
Leveraging Private Sector & Avoiding Duplication
Federal policies, including the NTTAA and 15 CFR Part 287, explicitly direct agencies to:
Use existing private-sector conformity assessment tools whenever practical.
Recognize accredited bodies instead of building parallel systems.
Engage in international cooperation to reduce duplicate testing.
➸ Example: Through Mutual Recognition Agreements (MRAs), a product tested in Europe by an accredited lab may not need retesting in the U.S., streamlining global market entry.
This integrated model promotes efficiency, regulatory credibility, and access to international markets, all while ensuring public safety and product integrity are not compromised.
Benefits, Costs & Challenges
Conformity assessment offers powerful advantages, market access, regulatory compliance, and public trust, but it also involves strategic decisions about cost, credibility, and long-term maintenance.
Understanding these trade-offs enables businesses to select the optimal path for their product or service.
Market Access & Consumer Confidence
One of the most immediate benefits of conformity assessment is market entry.
Many industries and jurisdictions will not accept products without documented proof of compliance.
Certification assures regulators, retailers, and end-users.
Testing and labeling help meet mandatory requirements and build voluntary trust
Marks from trusted organizations enhance a brand's reputation and foster consumer loyalty.
➸ Examples of recognized certification marks:
UL – Electrical and fire safety (widely required for U.S. retail shelves)
Energy Star – Energy efficiency and environmental performance
FCC Declaration – Wireless and electronic interference compliance
Without proper conformity documentation, businesses can face shipment holds, recalls, or even market bans, particularly in regulated sectors such as the medical, automotive, or telecommunications industries.
Cost vs. Risk Trade-Offs
Every conformity path involves a trade-off between cost, speed, and assurance.
Third-party testing and certification offer high credibility but involve greater cost and time.
First-party (self-declared) methods reduce cost and speed up launch, but may be insufficient for risk-sensitive markets.
The choice should reflect the product’s risk profile, market expectations, and regulatory requirements.
➸ Example: A children’s toy with small parts requires third-party testing for choking hazards, while a low-voltage USB cable might only require a supplier declaration for EMC.
Some industries also require independent certification, even if it’s not legally mandated, simply to gain retailer acceptance or win contracts.
In these cases, voluntary certification becomes a competitive advantage.
Keeping Up with Evolving Standards and Regulation Changes
Standards are updated frequently to reflect:
New technologies,
Evolving safety science,
Changes in environmental or cybersecurity risks,
Shifts in international regulatory expectations.
Manufacturers and conformity bodies must be proactive about:
Monitoring changes to relevant standards (ASTM, ISO, IEC, etc.),
Reviewing and updating documentation regularly,
Retesting or recertifying when critical standards are revised.
➸ Example: A company certified to an older version of ISO 13485 (medical device quality) must transition to the latest revision or risk losing certification and market access.
Lack of vigilance in this area can result in noncompliance, legal exposure, or business interruption.
Many companies assign compliance teams or utilize third-party monitoring services to stay current.
How Signify Helps You Navigate U.S. Conformity Assessment with Confidence
Signify is an AI compliance agent that helps manufacturers manage complex U.S. conformity assessment requirements with speed, accuracy, and control.
From FDA labeling rules to ISO documentation, Signify automates regulatory workflows, enabling teams to focus less on paperwork and more on delivering products.
Some of our key features include:
Automated Conformity Assessments: Instantly evaluate documentation and procedures against FDA, USDA, and ISO standards to identify compliance gaps before they become liabilities.
Smart Labeling Compliance: Validate labels for allergens, claims, formatting, and mandatory disclosures to avoid costly revisions and prevent launch delays.
Compliance Checklists with Remediation Guidance: Break down complex regulations into actionable tasks, with clear next steps and regulatory references.
Real-Time Regulatory Monitoring: Stay ahead of changes with automated alerts tailored to your industry, product category, and market.
Audit-Ready Documentation: Automatically generate structured, defensible records for FDA inspections, third-party certifications, and supplier reviews.
Global Regulation Harmonization: Align overlapping standards across multiple jurisdictions to reduce duplication and manual effort.
Regulatory Risk Tracker: Spot early warning signs in your supply chain or documentation before they escalate into compliance failures.
Supplier Verification: Confirm that suppliers meet U.S. safety, sourcing, and ethical standards through integrated checks and recordkeeping.
Traceable Evidence and Logs: Maintain full traceability with timestamped logs that link each regulatory requirement to its supporting evidence.
Try Signify today to see how AI-powered compliance agents can streamline conformity assessments, manage documentation, and keep you aligned with evolving U.S. regulations.
Did you know product recalls in the U.S. surged by 8% in the first quarter of 2024?
As safety concerns grow, regulators are tightening their grip on compliance through stricter conformity assessment practices.
In this guide, we’ll break down how conformity assessment works in the U.S., explore key regulatory frameworks, and share practical strategies to help your business stay compliant and competitive.
What is Conformity Assessment?
Conformity assessment refers to the processes used to evaluate whether products, services, systems, or personnel meet specific standards or requirements.
According to ISO/IEC 17000, it is the "demonstration that specified requirements relating to a product, process, system, person or body are fulfilled."
In the U.S. context, this includes testing, inspection, and certification activities that bridge the gap between standards and the marketplace, ensuring consistent safety, quality, and performance.

Why Focus on the U.S. Conformity Assessment System?
The U.S. system is decentralized and relies on collaboration between the public and private sectors.
Voluntary consensus standards play a major role, and government agencies often recognize or incorporate private-sector assessments.
Understanding how this hybrid system operates is crucial for ensuring compliance, facilitating market access, and fostering trust.
U.S. Standards and Regulatory Framework
1. Voluntary Consensus Standards & ANSI
The U.S. relies heavily on voluntary consensus standards (VCS) to support safety, interoperability, and compliance across industries.

Unlike countries with centralized standards bodies, the U.S. system is led by the private sector and decentralized, with coordination overseen by the American National Standards Institute (ANSI).
ANSI does not write standards itself. Instead, it:
Accredits standards developers,
Approves standards as American National Standards (ANS),
Ensures alignment with World Trade Organization Technical Barriers to Trade (WTO/TBT) principles.
➸ Key accredited developers include:
ASTM International: materials, construction, petroleum, environment
IEEE: electronics, telecommunications, power systems
ASME: boilers, piping, mechanical engineering
NFPA: fire protection and electrical safety
These organizations draft standards through open, consensus-driven processes, which are critical to product design, performance, and market access.
2. U.S. Government Regulatory Policies (NTTAA and OMB A-119)
To strengthen collaboration between the private sector and government, the U.S. adopted a policy framework that encourages federal reliance on voluntary standards and private-sector conformity assessment systems.
National Technology Transfer and Advancement Act (NTTAA)
Passed in 1995, the NTTAA (15 U.S.C. § 272) directs federal agencies to:
Use voluntary consensus standards (VCS) instead of government-unique standards whenever practical.
Participate in standard development activities to reflect public interests.
Report annually to the National Institute of Standards and Technology (NIST) on how VCSs are used.

➸ Example: Instead of writing a unique flame resistance test, a federal agency may adopt ASTM or NFPA fire standards.
This ensures that regulations are technology-neutral, globally aligned, and business-friendly.
OMB Circular A-119
Issued by the Office of Management and Budget (OMB), Circular A-119 provides specific guidance to federal agencies on implementing the NTTAA.

It instructs agencies to:
Prefer private-sector conformity assessment results when regulating or procuring products.
Avoid duplicating testing or certification processes that already exist in the market.
Engage early in standards development to shape outcomes aligned with regulatory needs.
➸ Impact: A product already certified by an ANSI-accredited body may not need retesting for federal procurement or compliance, saving businesses time and cost.
Together, the NTTAA and OMB A-119 promote regulatory efficiency, reduce technical barriers to trade, and foster innovation by integrating private sector expertise into federal processes.
Federal Guidance: 15 CFR Part 287
The Code of Federal Regulations Title 15, Part 287 provides overarching federal policy on how agencies should approach conformity assessment.
Issued by the National Institute of Standards and Technology (NIST), this regulation reinforces alignment with the NTTAA and OMB A-119, while also providing clarity on implementation.
Key Federal Objectives Under 15 CFR Part 287
NIST encourages agencies to adopt a coordinated, transparent, and risk-based approach to conformity assessment that serves the interests of both the public and private sectors.
➸ Agencies are guided to:
Minimize unnecessary regulatory burden on businesses.
Recognize and use accredited private-sector conformity assessment programs wherever appropriate.
Tailor conformity assessment activities based on risk, cost, and confidence level needed.
Promote transparency in the selection and application of assessments.
➸ Example: A low-risk product may only need a supplier’s declaration of conformity, while a high-risk product (like a medical device) may require third-party certification.
Federal-Private Sector Collaboration
15 CFR Part 287 also calls for:
Active collaboration with industry groups, trade associations, and standards bodies.
Efficient use of existing infrastructure, such as accredited labs and certification bodies.
Ongoing assessment of conformity practices to adapt to evolving technologies and risks.
➸ Impact: This framework reduces redundancy, enhances market confidence, and helps U.S. businesses meet both domestic and international requirements more efficiently.
U.S. National Conformity Assessment Principles (NCAP)
The U.S. National Conformity Assessment Principles (NCAP), developed by NIST, outline how conformity assessment should be applied across both public and private sectors.
These principles serve as a foundational policy framework to promote trust, reduce trade barriers, and ensure consistent application of conformity practices across industries.
Core Principles and WTO/TBT Alignment
NCAP aligns with the World Trade Organization (WTO) Agreement on Technical Barriers to Trade (TBT).
This alignment ensures that U.S. conformity practices support international trade while maintaining national regulatory objectives.

➸ Key principles include:
Avoiding unnecessary obstacles to trade.
Recognizing international equivalence of standards and assessments.
Treating domestic and foreign suppliers equally.
➸ Impact: A conformity certificate issued by an accredited lab in Europe may be accepted in the U.S. if it meets equivalent technical and accreditation criteria, reducing duplicative testing.
Purpose and Stakeholder Confidence
At its core, NCAP emphasizes that conformity assessment should deliver reliable, reproducible, and unbiased results, whether used by regulators, businesses, or consumers.
Confidence in results supports the enforcement of safety regulations, procurement decisions, and consumer trust.
Competency and impartiality of assessment bodies are essential for credibility.
➸ Example: When a testing lab is ISO/IEC 17025-accredited and follows NCAP principles, its results are more likely to be accepted across industries and borders.
Applicability Across Parties and Activities
NCAP is intentionally broad in scope. It applies not only to federal agencies but also to:
Private-sector certification bodies.
Inspection and testing labs.
Accreditation organizations.
Industry associations and trade groups.
The principles cover a wide range of conformity assessment activities, including:
Testing,
Inspection,
Certification,
Surveillance,
Accreditation.
➸ Benefit: This consistency ensures that different players in the conformity ecosystem operate with shared expectations and aligned procedures, reducing confusion and overlap.
Types of Conformity Assessment
Conformity assessment can be categorized into three levels of assurance based on the entity that performs the assessment.

Each type offers a different level of credibility, cost, and regulatory weight.
1. First-Party Assessment (Supplier’s Declaration of Conformity)
A first-party assessment is performed by the manufacturer or supplier themselves.
It’s often used in low-risk scenarios where internal controls are considered sufficient.
Involves self-testing, documentation, and a declaration that the product meets relevant standards.
Cost-effective and fast, but it relies entirely on internal processes and technical competency.
Common in industries where rapid innovation and short product cycles are prioritized.
➸ Example: A computer accessory manufacturer may self-declare compliance with FCC electromagnetic compatibility (EMC) requirements.
2. Second-Party Assessment (Customer-Driven Review)
A second-party assessment is conducted by the purchaser or client, usually a business vetting its suppliers or subcontractors.
Often includes on-site audits, documentation reviews, or product inspections.
Enables buyers to ensure their vendors meet quality, safety, or sustainability criteria.
Common in supply chain management and procurement settings.
➸ Example: A large retailer auditing its food suppliers for compliance with internal safety and labeling requirements.
3. Third-Party Assessment (Independent Certification)
A third-party assessment is conducted by a neutral, accredited organization with no stake in the outcome.
It is the most credible form of conformity assessment and is often required by law or industry regulations.
Provides objective, traceable, and standardized verification.
Recognized by regulators, buyers, and international markets.
Especially important for high-risk sectors, such as medical devices, electrical products, or construction materials.
➸ Example: A medical device certified by an FDA-accredited third-party review body or a consumer appliance certified by UL or Intertek for electrical safety.
Implementing U.S. Conformity Assessment
Conformity assessment in the U.S. follows a flexible, risk-based framework that aligns public oversight with private-sector expertise.
Agencies are encouraged to tailor their approaches based on risk, rely on accredited third-party bodies, and avoid redundant government processes.
Risk-Based Approach & Confidence/Regulation Spectrum
Federal policy encourages agencies to apply graduated levels of assessment depending on the product’s risk profile and the level of confidence required by regulators or consumers.
Assessment methods range from:
First-party declarations for low-risk, non-critical products.
Third-party certification for high-risk or safety-sensitive products.
Government-led review in highly regulated or national security sectors.
➸ Example: Toys may be self-declared for labeling accuracy, but children’s products with lead exposure risks require third-party testing under CPSC rules.
This model enables the U.S. system to strike a balance between oversight and innovation without imposing unnecessary regulatory burdens.
Federal Agency Use & Public/Private Alignment
Many federal agencies recognize or contract with private conformity assessment bodies instead of conducting assessments in-house.
This minimizes taxpayer costs while expanding access to qualified assessors.
➸ Example: The Occupational Safety and Health Administration (OSHA) recognizes Nationally Recognized Testing Laboratories (NRTLs), such as UL, to test electrical equipment, rather than operating its labs.
Other agencies, such as the EPA, FDA, and DOT, have similar frameworks that rely on private sector programs to meet their regulatory needs.
Leveraging Private Sector & Avoiding Duplication
Federal policies, including the NTTAA and 15 CFR Part 287, explicitly direct agencies to:
Use existing private-sector conformity assessment tools whenever practical.
Recognize accredited bodies instead of building parallel systems.
Engage in international cooperation to reduce duplicate testing.
➸ Example: Through Mutual Recognition Agreements (MRAs), a product tested in Europe by an accredited lab may not need retesting in the U.S., streamlining global market entry.
This integrated model promotes efficiency, regulatory credibility, and access to international markets, all while ensuring public safety and product integrity are not compromised.
Benefits, Costs & Challenges
Conformity assessment offers powerful advantages, market access, regulatory compliance, and public trust, but it also involves strategic decisions about cost, credibility, and long-term maintenance.
Understanding these trade-offs enables businesses to select the optimal path for their product or service.
Market Access & Consumer Confidence
One of the most immediate benefits of conformity assessment is market entry.
Many industries and jurisdictions will not accept products without documented proof of compliance.
Certification assures regulators, retailers, and end-users.
Testing and labeling help meet mandatory requirements and build voluntary trust
Marks from trusted organizations enhance a brand's reputation and foster consumer loyalty.
➸ Examples of recognized certification marks:
UL – Electrical and fire safety (widely required for U.S. retail shelves)
Energy Star – Energy efficiency and environmental performance
FCC Declaration – Wireless and electronic interference compliance
Without proper conformity documentation, businesses can face shipment holds, recalls, or even market bans, particularly in regulated sectors such as the medical, automotive, or telecommunications industries.
Cost vs. Risk Trade-Offs
Every conformity path involves a trade-off between cost, speed, and assurance.
Third-party testing and certification offer high credibility but involve greater cost and time.
First-party (self-declared) methods reduce cost and speed up launch, but may be insufficient for risk-sensitive markets.
The choice should reflect the product’s risk profile, market expectations, and regulatory requirements.
➸ Example: A children’s toy with small parts requires third-party testing for choking hazards, while a low-voltage USB cable might only require a supplier declaration for EMC.
Some industries also require independent certification, even if it’s not legally mandated, simply to gain retailer acceptance or win contracts.
In these cases, voluntary certification becomes a competitive advantage.
Keeping Up with Evolving Standards and Regulation Changes
Standards are updated frequently to reflect:
New technologies,
Evolving safety science,
Changes in environmental or cybersecurity risks,
Shifts in international regulatory expectations.
Manufacturers and conformity bodies must be proactive about:
Monitoring changes to relevant standards (ASTM, ISO, IEC, etc.),
Reviewing and updating documentation regularly,
Retesting or recertifying when critical standards are revised.
➸ Example: A company certified to an older version of ISO 13485 (medical device quality) must transition to the latest revision or risk losing certification and market access.
Lack of vigilance in this area can result in noncompliance, legal exposure, or business interruption.
Many companies assign compliance teams or utilize third-party monitoring services to stay current.
How Signify Helps You Navigate U.S. Conformity Assessment with Confidence
Signify is an AI compliance agent that helps manufacturers manage complex U.S. conformity assessment requirements with speed, accuracy, and control.
From FDA labeling rules to ISO documentation, Signify automates regulatory workflows, enabling teams to focus less on paperwork and more on delivering products.
Some of our key features include:
Automated Conformity Assessments: Instantly evaluate documentation and procedures against FDA, USDA, and ISO standards to identify compliance gaps before they become liabilities.
Smart Labeling Compliance: Validate labels for allergens, claims, formatting, and mandatory disclosures to avoid costly revisions and prevent launch delays.
Compliance Checklists with Remediation Guidance: Break down complex regulations into actionable tasks, with clear next steps and regulatory references.
Real-Time Regulatory Monitoring: Stay ahead of changes with automated alerts tailored to your industry, product category, and market.
Audit-Ready Documentation: Automatically generate structured, defensible records for FDA inspections, third-party certifications, and supplier reviews.
Global Regulation Harmonization: Align overlapping standards across multiple jurisdictions to reduce duplication and manual effort.
Regulatory Risk Tracker: Spot early warning signs in your supply chain or documentation before they escalate into compliance failures.
Supplier Verification: Confirm that suppliers meet U.S. safety, sourcing, and ethical standards through integrated checks and recordkeeping.
Traceable Evidence and Logs: Maintain full traceability with timestamped logs that link each regulatory requirement to its supporting evidence.
Try Signify today to see how AI-powered compliance agents can streamline conformity assessments, manage documentation, and keep you aligned with evolving U.S. regulations.
Did you know product recalls in the U.S. surged by 8% in the first quarter of 2024?
As safety concerns grow, regulators are tightening their grip on compliance through stricter conformity assessment practices.
In this guide, we’ll break down how conformity assessment works in the U.S., explore key regulatory frameworks, and share practical strategies to help your business stay compliant and competitive.
What is Conformity Assessment?
Conformity assessment refers to the processes used to evaluate whether products, services, systems, or personnel meet specific standards or requirements.
According to ISO/IEC 17000, it is the "demonstration that specified requirements relating to a product, process, system, person or body are fulfilled."
In the U.S. context, this includes testing, inspection, and certification activities that bridge the gap between standards and the marketplace, ensuring consistent safety, quality, and performance.

Why Focus on the U.S. Conformity Assessment System?
The U.S. system is decentralized and relies on collaboration between the public and private sectors.
Voluntary consensus standards play a major role, and government agencies often recognize or incorporate private-sector assessments.
Understanding how this hybrid system operates is crucial for ensuring compliance, facilitating market access, and fostering trust.
U.S. Standards and Regulatory Framework
1. Voluntary Consensus Standards & ANSI
The U.S. relies heavily on voluntary consensus standards (VCS) to support safety, interoperability, and compliance across industries.

Unlike countries with centralized standards bodies, the U.S. system is led by the private sector and decentralized, with coordination overseen by the American National Standards Institute (ANSI).
ANSI does not write standards itself. Instead, it:
Accredits standards developers,
Approves standards as American National Standards (ANS),
Ensures alignment with World Trade Organization Technical Barriers to Trade (WTO/TBT) principles.
➸ Key accredited developers include:
ASTM International: materials, construction, petroleum, environment
IEEE: electronics, telecommunications, power systems
ASME: boilers, piping, mechanical engineering
NFPA: fire protection and electrical safety
These organizations draft standards through open, consensus-driven processes, which are critical to product design, performance, and market access.
2. U.S. Government Regulatory Policies (NTTAA and OMB A-119)
To strengthen collaboration between the private sector and government, the U.S. adopted a policy framework that encourages federal reliance on voluntary standards and private-sector conformity assessment systems.
National Technology Transfer and Advancement Act (NTTAA)
Passed in 1995, the NTTAA (15 U.S.C. § 272) directs federal agencies to:
Use voluntary consensus standards (VCS) instead of government-unique standards whenever practical.
Participate in standard development activities to reflect public interests.
Report annually to the National Institute of Standards and Technology (NIST) on how VCSs are used.

➸ Example: Instead of writing a unique flame resistance test, a federal agency may adopt ASTM or NFPA fire standards.
This ensures that regulations are technology-neutral, globally aligned, and business-friendly.
OMB Circular A-119
Issued by the Office of Management and Budget (OMB), Circular A-119 provides specific guidance to federal agencies on implementing the NTTAA.

It instructs agencies to:
Prefer private-sector conformity assessment results when regulating or procuring products.
Avoid duplicating testing or certification processes that already exist in the market.
Engage early in standards development to shape outcomes aligned with regulatory needs.
➸ Impact: A product already certified by an ANSI-accredited body may not need retesting for federal procurement or compliance, saving businesses time and cost.
Together, the NTTAA and OMB A-119 promote regulatory efficiency, reduce technical barriers to trade, and foster innovation by integrating private sector expertise into federal processes.
Federal Guidance: 15 CFR Part 287
The Code of Federal Regulations Title 15, Part 287 provides overarching federal policy on how agencies should approach conformity assessment.
Issued by the National Institute of Standards and Technology (NIST), this regulation reinforces alignment with the NTTAA and OMB A-119, while also providing clarity on implementation.
Key Federal Objectives Under 15 CFR Part 287
NIST encourages agencies to adopt a coordinated, transparent, and risk-based approach to conformity assessment that serves the interests of both the public and private sectors.
➸ Agencies are guided to:
Minimize unnecessary regulatory burden on businesses.
Recognize and use accredited private-sector conformity assessment programs wherever appropriate.
Tailor conformity assessment activities based on risk, cost, and confidence level needed.
Promote transparency in the selection and application of assessments.
➸ Example: A low-risk product may only need a supplier’s declaration of conformity, while a high-risk product (like a medical device) may require third-party certification.
Federal-Private Sector Collaboration
15 CFR Part 287 also calls for:
Active collaboration with industry groups, trade associations, and standards bodies.
Efficient use of existing infrastructure, such as accredited labs and certification bodies.
Ongoing assessment of conformity practices to adapt to evolving technologies and risks.
➸ Impact: This framework reduces redundancy, enhances market confidence, and helps U.S. businesses meet both domestic and international requirements more efficiently.
U.S. National Conformity Assessment Principles (NCAP)
The U.S. National Conformity Assessment Principles (NCAP), developed by NIST, outline how conformity assessment should be applied across both public and private sectors.
These principles serve as a foundational policy framework to promote trust, reduce trade barriers, and ensure consistent application of conformity practices across industries.
Core Principles and WTO/TBT Alignment
NCAP aligns with the World Trade Organization (WTO) Agreement on Technical Barriers to Trade (TBT).
This alignment ensures that U.S. conformity practices support international trade while maintaining national regulatory objectives.

➸ Key principles include:
Avoiding unnecessary obstacles to trade.
Recognizing international equivalence of standards and assessments.
Treating domestic and foreign suppliers equally.
➸ Impact: A conformity certificate issued by an accredited lab in Europe may be accepted in the U.S. if it meets equivalent technical and accreditation criteria, reducing duplicative testing.
Purpose and Stakeholder Confidence
At its core, NCAP emphasizes that conformity assessment should deliver reliable, reproducible, and unbiased results, whether used by regulators, businesses, or consumers.
Confidence in results supports the enforcement of safety regulations, procurement decisions, and consumer trust.
Competency and impartiality of assessment bodies are essential for credibility.
➸ Example: When a testing lab is ISO/IEC 17025-accredited and follows NCAP principles, its results are more likely to be accepted across industries and borders.
Applicability Across Parties and Activities
NCAP is intentionally broad in scope. It applies not only to federal agencies but also to:
Private-sector certification bodies.
Inspection and testing labs.
Accreditation organizations.
Industry associations and trade groups.
The principles cover a wide range of conformity assessment activities, including:
Testing,
Inspection,
Certification,
Surveillance,
Accreditation.
➸ Benefit: This consistency ensures that different players in the conformity ecosystem operate with shared expectations and aligned procedures, reducing confusion and overlap.
Types of Conformity Assessment
Conformity assessment can be categorized into three levels of assurance based on the entity that performs the assessment.

Each type offers a different level of credibility, cost, and regulatory weight.
1. First-Party Assessment (Supplier’s Declaration of Conformity)
A first-party assessment is performed by the manufacturer or supplier themselves.
It’s often used in low-risk scenarios where internal controls are considered sufficient.
Involves self-testing, documentation, and a declaration that the product meets relevant standards.
Cost-effective and fast, but it relies entirely on internal processes and technical competency.
Common in industries where rapid innovation and short product cycles are prioritized.
➸ Example: A computer accessory manufacturer may self-declare compliance with FCC electromagnetic compatibility (EMC) requirements.
2. Second-Party Assessment (Customer-Driven Review)
A second-party assessment is conducted by the purchaser or client, usually a business vetting its suppliers or subcontractors.
Often includes on-site audits, documentation reviews, or product inspections.
Enables buyers to ensure their vendors meet quality, safety, or sustainability criteria.
Common in supply chain management and procurement settings.
➸ Example: A large retailer auditing its food suppliers for compliance with internal safety and labeling requirements.
3. Third-Party Assessment (Independent Certification)
A third-party assessment is conducted by a neutral, accredited organization with no stake in the outcome.
It is the most credible form of conformity assessment and is often required by law or industry regulations.
Provides objective, traceable, and standardized verification.
Recognized by regulators, buyers, and international markets.
Especially important for high-risk sectors, such as medical devices, electrical products, or construction materials.
➸ Example: A medical device certified by an FDA-accredited third-party review body or a consumer appliance certified by UL or Intertek for electrical safety.
Implementing U.S. Conformity Assessment
Conformity assessment in the U.S. follows a flexible, risk-based framework that aligns public oversight with private-sector expertise.
Agencies are encouraged to tailor their approaches based on risk, rely on accredited third-party bodies, and avoid redundant government processes.
Risk-Based Approach & Confidence/Regulation Spectrum
Federal policy encourages agencies to apply graduated levels of assessment depending on the product’s risk profile and the level of confidence required by regulators or consumers.
Assessment methods range from:
First-party declarations for low-risk, non-critical products.
Third-party certification for high-risk or safety-sensitive products.
Government-led review in highly regulated or national security sectors.
➸ Example: Toys may be self-declared for labeling accuracy, but children’s products with lead exposure risks require third-party testing under CPSC rules.
This model enables the U.S. system to strike a balance between oversight and innovation without imposing unnecessary regulatory burdens.
Federal Agency Use & Public/Private Alignment
Many federal agencies recognize or contract with private conformity assessment bodies instead of conducting assessments in-house.
This minimizes taxpayer costs while expanding access to qualified assessors.
➸ Example: The Occupational Safety and Health Administration (OSHA) recognizes Nationally Recognized Testing Laboratories (NRTLs), such as UL, to test electrical equipment, rather than operating its labs.
Other agencies, such as the EPA, FDA, and DOT, have similar frameworks that rely on private sector programs to meet their regulatory needs.
Leveraging Private Sector & Avoiding Duplication
Federal policies, including the NTTAA and 15 CFR Part 287, explicitly direct agencies to:
Use existing private-sector conformity assessment tools whenever practical.
Recognize accredited bodies instead of building parallel systems.
Engage in international cooperation to reduce duplicate testing.
➸ Example: Through Mutual Recognition Agreements (MRAs), a product tested in Europe by an accredited lab may not need retesting in the U.S., streamlining global market entry.
This integrated model promotes efficiency, regulatory credibility, and access to international markets, all while ensuring public safety and product integrity are not compromised.
Benefits, Costs & Challenges
Conformity assessment offers powerful advantages, market access, regulatory compliance, and public trust, but it also involves strategic decisions about cost, credibility, and long-term maintenance.
Understanding these trade-offs enables businesses to select the optimal path for their product or service.
Market Access & Consumer Confidence
One of the most immediate benefits of conformity assessment is market entry.
Many industries and jurisdictions will not accept products without documented proof of compliance.
Certification assures regulators, retailers, and end-users.
Testing and labeling help meet mandatory requirements and build voluntary trust
Marks from trusted organizations enhance a brand's reputation and foster consumer loyalty.
➸ Examples of recognized certification marks:
UL – Electrical and fire safety (widely required for U.S. retail shelves)
Energy Star – Energy efficiency and environmental performance
FCC Declaration – Wireless and electronic interference compliance
Without proper conformity documentation, businesses can face shipment holds, recalls, or even market bans, particularly in regulated sectors such as the medical, automotive, or telecommunications industries.
Cost vs. Risk Trade-Offs
Every conformity path involves a trade-off between cost, speed, and assurance.
Third-party testing and certification offer high credibility but involve greater cost and time.
First-party (self-declared) methods reduce cost and speed up launch, but may be insufficient for risk-sensitive markets.
The choice should reflect the product’s risk profile, market expectations, and regulatory requirements.
➸ Example: A children’s toy with small parts requires third-party testing for choking hazards, while a low-voltage USB cable might only require a supplier declaration for EMC.
Some industries also require independent certification, even if it’s not legally mandated, simply to gain retailer acceptance or win contracts.
In these cases, voluntary certification becomes a competitive advantage.
Keeping Up with Evolving Standards and Regulation Changes
Standards are updated frequently to reflect:
New technologies,
Evolving safety science,
Changes in environmental or cybersecurity risks,
Shifts in international regulatory expectations.
Manufacturers and conformity bodies must be proactive about:
Monitoring changes to relevant standards (ASTM, ISO, IEC, etc.),
Reviewing and updating documentation regularly,
Retesting or recertifying when critical standards are revised.
➸ Example: A company certified to an older version of ISO 13485 (medical device quality) must transition to the latest revision or risk losing certification and market access.
Lack of vigilance in this area can result in noncompliance, legal exposure, or business interruption.
Many companies assign compliance teams or utilize third-party monitoring services to stay current.
How Signify Helps You Navigate U.S. Conformity Assessment with Confidence
Signify is an AI compliance agent that helps manufacturers manage complex U.S. conformity assessment requirements with speed, accuracy, and control.
From FDA labeling rules to ISO documentation, Signify automates regulatory workflows, enabling teams to focus less on paperwork and more on delivering products.
Some of our key features include:
Automated Conformity Assessments: Instantly evaluate documentation and procedures against FDA, USDA, and ISO standards to identify compliance gaps before they become liabilities.
Smart Labeling Compliance: Validate labels for allergens, claims, formatting, and mandatory disclosures to avoid costly revisions and prevent launch delays.
Compliance Checklists with Remediation Guidance: Break down complex regulations into actionable tasks, with clear next steps and regulatory references.
Real-Time Regulatory Monitoring: Stay ahead of changes with automated alerts tailored to your industry, product category, and market.
Audit-Ready Documentation: Automatically generate structured, defensible records for FDA inspections, third-party certifications, and supplier reviews.
Global Regulation Harmonization: Align overlapping standards across multiple jurisdictions to reduce duplication and manual effort.
Regulatory Risk Tracker: Spot early warning signs in your supply chain or documentation before they escalate into compliance failures.
Supplier Verification: Confirm that suppliers meet U.S. safety, sourcing, and ethical standards through integrated checks and recordkeeping.
Traceable Evidence and Logs: Maintain full traceability with timestamped logs that link each regulatory requirement to its supporting evidence.
Try Signify today to see how AI-powered compliance agents can streamline conformity assessments, manage documentation, and keep you aligned with evolving U.S. regulations.
The information presented is for educational and informational purposes only and should not be construed as legal, regulatory, or professional advice. Organizations should consult with qualified legal and compliance professionals for guidance specific to their circumstances.
Conformity Assessment US - Everything You Need To Know
Conformity Assessment US - Everything You Need To Know
Jun 27, 2025