Jan 21, 2026
Articles
Jan 21, 2026
Articles
Jan 21, 2026
Articles
Why Food Formula Validation Matters: Insights for Food Manufacturers

Martín Ramírez

Martín Ramírez

Martín Ramírez

Introduction
Behind every food product label lies a world of complexity. Food formula validation is the rigorous process that ensures every ingredient, claim, and nutritional value meets the highest standards of safety and regulatory compliance. For compliance professionals, this work is both a science and an art—balancing evolving regulations, consumer safety, and brand reputation.
The stakes could not be higher. A single misstep in the formula validation process can trigger regulatory action, product recalls, or even endanger public health. Yet, the professionals who carry out food formula validation often work behind the scenes, navigating a labyrinth of ingredient compliance, food safety verification, and formula review requirements.
This comprehensive guide will demystify the hidden complexity behind food formula validation. We will explore the manual processes that have long defined the industry, the expertise required to navigate them, and the far-reaching impact of validation failures. We will also look ahead to the future, where AI-powered compliance solutions are transforming how brands bring safe, compliant products to market. Whether you are a regulatory affairs specialist, a quality manager, or a business leader, this guide will equip you to meet today’s challenges and prepare for tomorrow’s demands.
What Is Food Formula Validation?
Definition and Scope
Food formula validation is the systematic review of a food product’s formula to ensure it meets all regulatory requirements before reaching the market. This process encompasses ingredient legality, concentration limits, labeling accuracy, and the substantiation of marketing claims. Unlike quality control or safety testing, which focus on finished products, formula validation scrutinizes the composition and documentation of each product at the earliest stages.
The formula validation process is a cornerstone of food product compliance. It involves a detailed food product formula review, where each ingredient is assessed for compliance with federal, state, and international standards. Ingredient validation ensures that every component is legal, safe, and accurately represented.
The Regulatory Landscape
The regulatory environment for food formula validation is complex and ever-changing. In the United States, the Food and Drug Administration (FDA) governs food ingredients, additives, and labeling through Title 21 of the Code of Federal Regulations (CFR). State-level requirements, such as California’s Proposition 65, add further complexity, while products intended for international markets must comply with foreign standards and import restrictions.
Each product category—conventional foods, dietary supplements, beverages, and functional foods—has its own regulatory nuances. For example, dietary supplements are regulated under the Dietary Supplement Health and Education Act (DSHEA), while functional foods may face additional scrutiny regarding health claims.
What Gets Validated
The formula validation process covers several critical areas:
Ingredient identity and specifications: Ensuring each component is accurately described and meets regulatory definitions.
Concentration levels and daily intake calculations: Verifying that ingredient amounts are within legal and safe limits.
Allergen declarations: Confirming that all potential allergens are properly disclosed.
Nutrient content and nutrition facts accuracy: Ensuring that nutritional information is correct and substantiated.
Marketing claims and their regulatory basis: Validating that all claims are supported by scientific evidence and permitted by law.
Label compliance: Reviewing required statements, formatting, and placement to meet regulatory standards.
The Manual Formula Validation Process
The formula validation process begins with document collection, where the reviewer gathers all relevant specifications, certificates of analysis, and supplier documentation needed to evaluate the product. With materials in hand, the reviewer works through each ingredient individually, cross-referencing components against regulatory databases to confirm legality and compliance status. This ingredient-level review feeds directly into concentration calculations, where the reviewer verifies that levels fall within permitted ranges after accounting for serving sizes and daily intake limits.
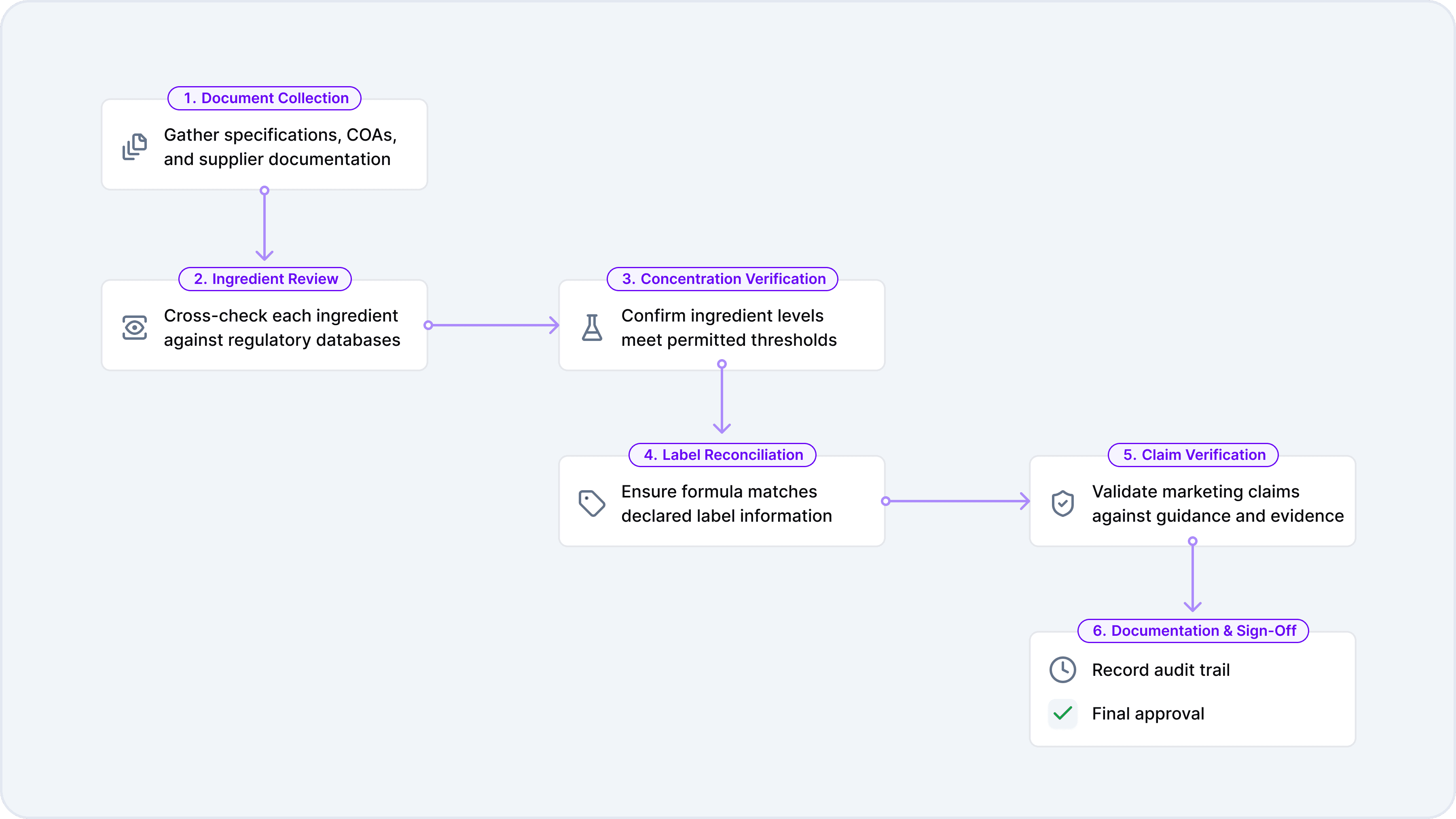
Once the formula itself checks out, attention shifts to the label. Label reconciliation ensures the formula matches what's declared on packaging and that all required information appears correctly. Any marketing claims are then verified to confirm they're supported by regulatory guidance and scientific evidence. The process concludes with documentation and sign-off, creating the audit trails and approval records that demonstrate due diligence and support future audits or regulatory inquiries.
Tools and Resources Used in Manual Review
Compliance professionals rely on a range of resources, including:
FDA databases (GRAS notices, food additive status lists, EAFUS)
CFR references and guidance documents
Proprietary ingredient databases
Spreadsheets and document management systems
Institutional knowledge and reference libraries
Time and Resource Requirements
The time required for a formula review varies widely. Simple products may require only a few hours, while complex formulations can take days or even weeks. Advanced degrees in food science or regulatory affairs are often necessary, and review cycles may involve multiple rounds of revision and documentation. The burden of record-keeping and audit trail maintenance adds further complexity.
The Expertise Behind the Process
Formula validation demands specialized knowledge. Regulatory affairs professionals must stay current with evolving regulations, interpret complex guidance, and apply scientific judgment to novel ingredients and claims. Ongoing training and professional development are essential to maintain expertise and ensure compliance.
Risks of Manual Formula Validation
Human Error in Complex Reviews
Manual reviews are susceptible to human error, especially when cross-referencing multiple regulations. Version control issues, transcription mistakes, and calculation errors can all compromise the integrity of the validation process. High-volume environments increase the risk of fatigue-related mistakes.
Knowledge Gaps and Regulatory Blind Spots
Keeping up with regulatory changes is a constant challenge. State-level requirements and international regulations may be overlooked, and novel ingredients can present ambiguous compliance questions.
Inconsistency Across Reviewers
Different reviewers may interpret regulations differently, leading to inconsistent outcomes. Lack of standardized processes and reliance on institutional knowledge can result in gaps when experienced staff leave the organization.
Documentation and Audit Trail Gaps
Incomplete records of review rationale make it difficult to demonstrate due diligence. Reconstructing review history during audits or recalls can be challenging, exposing organizations to regulatory risk.
Real-World Consequences of Validation Failures
Validation failures can lead to warning letters, enforcement actions, product recalls, and litigation. Recent FDA warning letters highlight the serious consequences of non-compliance, including financial losses and reputational damage. For example, in 2023, the FDA issued multiple warning letters to food manufacturers for mislabeling and undeclared allergens, resulting in costly recalls and heightened scrutiny.
The True Cost of Manual Validation
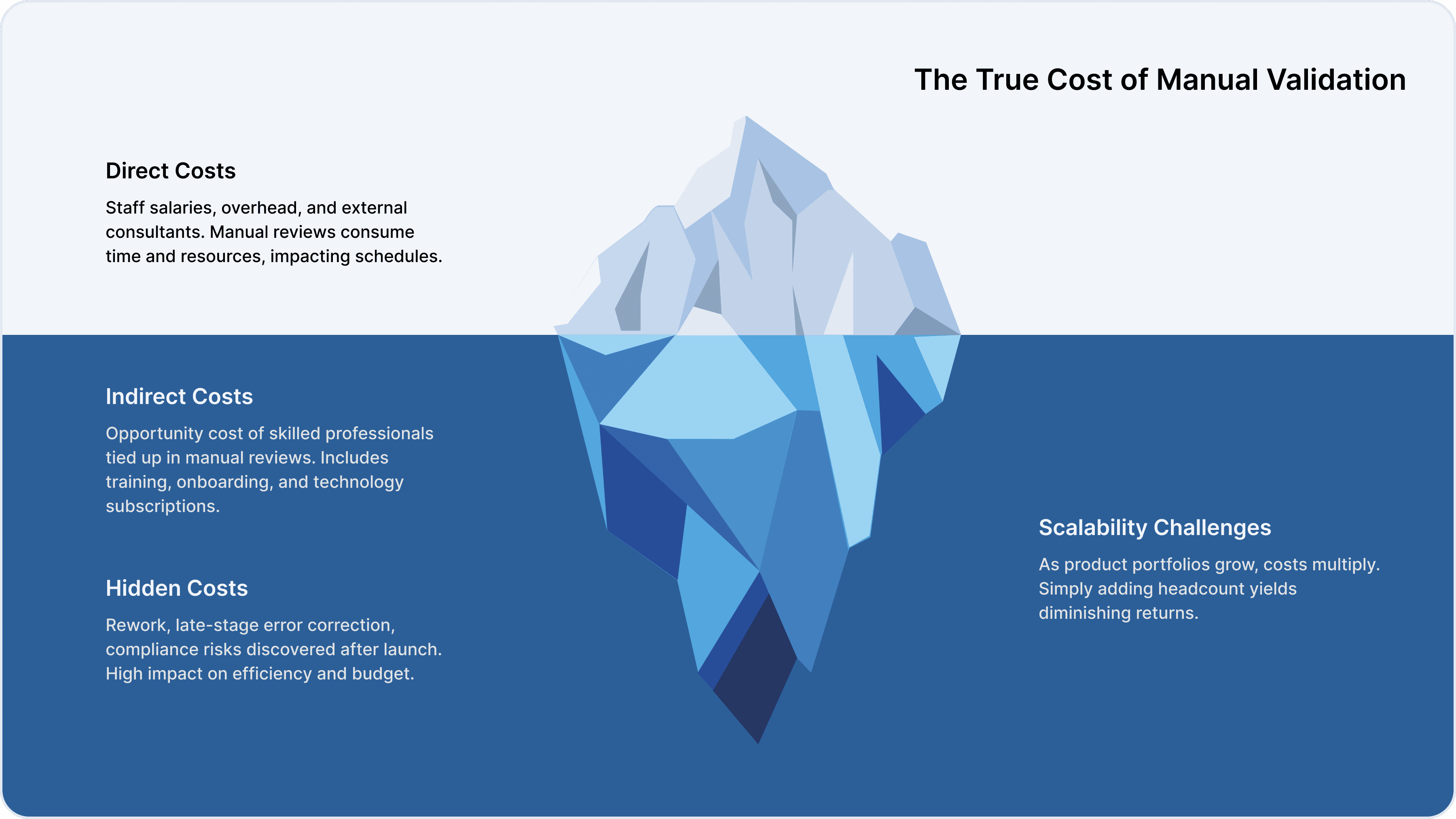
Direct Operating Costs
Manual validation requires a significant investment in specialized staff, resulting in high salaries and overhead costs. Extended review cycles can delay time-to-market, and external consultants may be needed for complex formulas.
Indirect and Hidden Costs
Opportunity costs arise when skilled professionals are tied up in manual reviews rather than strategic initiatives. Training and onboarding expenses, technology subscriptions, and the cost of rework when errors are discovered late in development all add to the total cost.
Scalability Challenges
As product portfolios grow, costs multiply. Seasonal launches and capacity crunches strain resources, and simply adding headcount yields diminishing returns without process improvements.
Stakeholder Impact: How Validation Failures Ripple Across the Supply Chain
Impact on Brands
Regulatory enforcement, warning letters, and recalls can damage brand reputation and strain retailer relationships. Consumer trust is eroded, and financial exposure from recalls and litigation can be significant. A single validation failure can have lasting consequences, as seen in high-profile industry cases.
Example: In 2022, a major snack brand faced a nationwide recall due to undeclared allergens, resulting in millions of dollars in lost sales and a significant drop in consumer trust.
Impact on Manufacturers
Production delays, waste from non-compliant batches, and damaged relationships with brand partners are common outcomes. Manufacturers may also face liability exposure and complications during audits and certifications.
Impact on Contract Manufacturing Partners
Co-packers and third-party manufacturers often have limited visibility into formula development decisions but share liability for compliance failures. Managing validation across multiple clients increases complexity and documentation burdens.
Impact on Retailers
Retailers face shelf liability for non-compliant products, regulatory scrutiny, and supply chain disruptions when products are pulled. Private label products present unique validation challenges, and consumer complaints must be managed effectively.
Impact on Consumers
Consumers are exposed to health and safety risks from undeclared allergens or unsafe ingredients. Misleading claims can affect purchasing decisions and health outcomes, particularly for vulnerable populations such as those with allergies, dietary restrictions, or children.
Modernizing Formula Validation with AI
The Case for Technology-Enabled Validation
The volume and complexity of modern food portfolios have outpaced manual validation capacity. Regulatory changes are accelerating, and skilled compliance professionals are in short supply. AI-powered solutions offer the opportunity to elevate human expertise by automating repetitive tasks and enabling strategic focus.
How AI Transforms the Validation Process
AI systems can automatically cross-reference formulas against comprehensive regulatory databases, flagging potential compliance issues in real time. They apply consistent regulatory logic across all reviews, instantly identify state-level and international requirements, and continuously monitor for regulatory changes.
Business Value of AI-Powered Validation
Speed: Review cycles are reduced from days to minutes.
Accuracy: Manual lookups and calculation errors are eliminated.
Consistency: Rigorous standards are applied to every formula.
Scalability: Portfolio growth is managed without proportional increases in headcount.
Auditability: Review logic and decisions are automatically documented.
Strategic reallocation: Compliance professionals are freed for higher-value work.
The Human-AI Partnership Model
AI handles repetitive cross-referencing, while humans apply judgment and strategy. Compliance professionals can focus on novel questions and risk decisions, elevating their role from document reviewer to strategic advisor. Domain expertise remains essential in an AI-augmented workflow.
Building a Modern Validation Program
Evaluating Your Current State
Assess your validation process maturity by benchmarking review times, error rates, and identifying warning signs of overburdened manual processes. Are review cycles consistently delayed? Are errors or inconsistencies surfacing during audits or recalls? These are signals that modernization is needed.
Key Capabilities to Look For
Seek solutions with comprehensive regulatory coverage, transparent reasoning, audit trails, integration with existing workflows, and flexibility for multiple product categories. The right system should support both federal and state requirements and international standards for global brands.
Change Management Considerations
Secure buy-in from compliance teams by emphasizing augmentation, not replacement. Pilot programs and proof-of-concept approaches can demonstrate value, and ROI should be measured and communicated clearly. Change management is critical to ensure a smooth transition and lasting adoption.
The Future of Food Formula Validation
Regulatory complexity will continue to increase, and consumer expectations for transparency are rising. Supply chain traceability demands are expanding, making efficient, reliable compliance a competitive advantage. Forward-thinking organizations are positioning compliance as a strategic function rather than a cost center. Those who modernize their formula validation process will be best equipped to protect consumers, brands, and the broader supply chain.
Conclusion
Food formula validation is a cornerstone of food safety and compliance, protecting consumers, brands, and the broader supply chain. The dedication of compliance professionals ensures that every product meets rigorous standards, even as the landscape grows more complex. By embracing modern, AI-powered solutions, organizations can achieve greater efficiency and accuracy without sacrificing the rigor that underpins consumer trust. Compliance done well is not just a regulatory requirement—it is a vital safeguard for everyone involved.
Introduction
Behind every food product label lies a world of complexity. Food formula validation is the rigorous process that ensures every ingredient, claim, and nutritional value meets the highest standards of safety and regulatory compliance. For compliance professionals, this work is both a science and an art—balancing evolving regulations, consumer safety, and brand reputation.
The stakes could not be higher. A single misstep in the formula validation process can trigger regulatory action, product recalls, or even endanger public health. Yet, the professionals who carry out food formula validation often work behind the scenes, navigating a labyrinth of ingredient compliance, food safety verification, and formula review requirements.
This comprehensive guide will demystify the hidden complexity behind food formula validation. We will explore the manual processes that have long defined the industry, the expertise required to navigate them, and the far-reaching impact of validation failures. We will also look ahead to the future, where AI-powered compliance solutions are transforming how brands bring safe, compliant products to market. Whether you are a regulatory affairs specialist, a quality manager, or a business leader, this guide will equip you to meet today’s challenges and prepare for tomorrow’s demands.
What Is Food Formula Validation?
Definition and Scope
Food formula validation is the systematic review of a food product’s formula to ensure it meets all regulatory requirements before reaching the market. This process encompasses ingredient legality, concentration limits, labeling accuracy, and the substantiation of marketing claims. Unlike quality control or safety testing, which focus on finished products, formula validation scrutinizes the composition and documentation of each product at the earliest stages.
The formula validation process is a cornerstone of food product compliance. It involves a detailed food product formula review, where each ingredient is assessed for compliance with federal, state, and international standards. Ingredient validation ensures that every component is legal, safe, and accurately represented.
The Regulatory Landscape
The regulatory environment for food formula validation is complex and ever-changing. In the United States, the Food and Drug Administration (FDA) governs food ingredients, additives, and labeling through Title 21 of the Code of Federal Regulations (CFR). State-level requirements, such as California’s Proposition 65, add further complexity, while products intended for international markets must comply with foreign standards and import restrictions.
Each product category—conventional foods, dietary supplements, beverages, and functional foods—has its own regulatory nuances. For example, dietary supplements are regulated under the Dietary Supplement Health and Education Act (DSHEA), while functional foods may face additional scrutiny regarding health claims.
What Gets Validated
The formula validation process covers several critical areas:
Ingredient identity and specifications: Ensuring each component is accurately described and meets regulatory definitions.
Concentration levels and daily intake calculations: Verifying that ingredient amounts are within legal and safe limits.
Allergen declarations: Confirming that all potential allergens are properly disclosed.
Nutrient content and nutrition facts accuracy: Ensuring that nutritional information is correct and substantiated.
Marketing claims and their regulatory basis: Validating that all claims are supported by scientific evidence and permitted by law.
Label compliance: Reviewing required statements, formatting, and placement to meet regulatory standards.
The Manual Formula Validation Process
The formula validation process begins with document collection, where the reviewer gathers all relevant specifications, certificates of analysis, and supplier documentation needed to evaluate the product. With materials in hand, the reviewer works through each ingredient individually, cross-referencing components against regulatory databases to confirm legality and compliance status. This ingredient-level review feeds directly into concentration calculations, where the reviewer verifies that levels fall within permitted ranges after accounting for serving sizes and daily intake limits.

Once the formula itself checks out, attention shifts to the label. Label reconciliation ensures the formula matches what's declared on packaging and that all required information appears correctly. Any marketing claims are then verified to confirm they're supported by regulatory guidance and scientific evidence. The process concludes with documentation and sign-off, creating the audit trails and approval records that demonstrate due diligence and support future audits or regulatory inquiries.
Tools and Resources Used in Manual Review
Compliance professionals rely on a range of resources, including:
FDA databases (GRAS notices, food additive status lists, EAFUS)
CFR references and guidance documents
Proprietary ingredient databases
Spreadsheets and document management systems
Institutional knowledge and reference libraries
Time and Resource Requirements
The time required for a formula review varies widely. Simple products may require only a few hours, while complex formulations can take days or even weeks. Advanced degrees in food science or regulatory affairs are often necessary, and review cycles may involve multiple rounds of revision and documentation. The burden of record-keeping and audit trail maintenance adds further complexity.
The Expertise Behind the Process
Formula validation demands specialized knowledge. Regulatory affairs professionals must stay current with evolving regulations, interpret complex guidance, and apply scientific judgment to novel ingredients and claims. Ongoing training and professional development are essential to maintain expertise and ensure compliance.
Risks of Manual Formula Validation
Human Error in Complex Reviews
Manual reviews are susceptible to human error, especially when cross-referencing multiple regulations. Version control issues, transcription mistakes, and calculation errors can all compromise the integrity of the validation process. High-volume environments increase the risk of fatigue-related mistakes.
Knowledge Gaps and Regulatory Blind Spots
Keeping up with regulatory changes is a constant challenge. State-level requirements and international regulations may be overlooked, and novel ingredients can present ambiguous compliance questions.
Inconsistency Across Reviewers
Different reviewers may interpret regulations differently, leading to inconsistent outcomes. Lack of standardized processes and reliance on institutional knowledge can result in gaps when experienced staff leave the organization.
Documentation and Audit Trail Gaps
Incomplete records of review rationale make it difficult to demonstrate due diligence. Reconstructing review history during audits or recalls can be challenging, exposing organizations to regulatory risk.
Real-World Consequences of Validation Failures
Validation failures can lead to warning letters, enforcement actions, product recalls, and litigation. Recent FDA warning letters highlight the serious consequences of non-compliance, including financial losses and reputational damage. For example, in 2023, the FDA issued multiple warning letters to food manufacturers for mislabeling and undeclared allergens, resulting in costly recalls and heightened scrutiny.
The True Cost of Manual Validation

Direct Operating Costs
Manual validation requires a significant investment in specialized staff, resulting in high salaries and overhead costs. Extended review cycles can delay time-to-market, and external consultants may be needed for complex formulas.
Indirect and Hidden Costs
Opportunity costs arise when skilled professionals are tied up in manual reviews rather than strategic initiatives. Training and onboarding expenses, technology subscriptions, and the cost of rework when errors are discovered late in development all add to the total cost.
Scalability Challenges
As product portfolios grow, costs multiply. Seasonal launches and capacity crunches strain resources, and simply adding headcount yields diminishing returns without process improvements.
Stakeholder Impact: How Validation Failures Ripple Across the Supply Chain
Impact on Brands
Regulatory enforcement, warning letters, and recalls can damage brand reputation and strain retailer relationships. Consumer trust is eroded, and financial exposure from recalls and litigation can be significant. A single validation failure can have lasting consequences, as seen in high-profile industry cases.
Example: In 2022, a major snack brand faced a nationwide recall due to undeclared allergens, resulting in millions of dollars in lost sales and a significant drop in consumer trust.
Impact on Manufacturers
Production delays, waste from non-compliant batches, and damaged relationships with brand partners are common outcomes. Manufacturers may also face liability exposure and complications during audits and certifications.
Impact on Contract Manufacturing Partners
Co-packers and third-party manufacturers often have limited visibility into formula development decisions but share liability for compliance failures. Managing validation across multiple clients increases complexity and documentation burdens.
Impact on Retailers
Retailers face shelf liability for non-compliant products, regulatory scrutiny, and supply chain disruptions when products are pulled. Private label products present unique validation challenges, and consumer complaints must be managed effectively.
Impact on Consumers
Consumers are exposed to health and safety risks from undeclared allergens or unsafe ingredients. Misleading claims can affect purchasing decisions and health outcomes, particularly for vulnerable populations such as those with allergies, dietary restrictions, or children.
Modernizing Formula Validation with AI
The Case for Technology-Enabled Validation
The volume and complexity of modern food portfolios have outpaced manual validation capacity. Regulatory changes are accelerating, and skilled compliance professionals are in short supply. AI-powered solutions offer the opportunity to elevate human expertise by automating repetitive tasks and enabling strategic focus.
How AI Transforms the Validation Process
AI systems can automatically cross-reference formulas against comprehensive regulatory databases, flagging potential compliance issues in real time. They apply consistent regulatory logic across all reviews, instantly identify state-level and international requirements, and continuously monitor for regulatory changes.
Business Value of AI-Powered Validation
Speed: Review cycles are reduced from days to minutes.
Accuracy: Manual lookups and calculation errors are eliminated.
Consistency: Rigorous standards are applied to every formula.
Scalability: Portfolio growth is managed without proportional increases in headcount.
Auditability: Review logic and decisions are automatically documented.
Strategic reallocation: Compliance professionals are freed for higher-value work.
The Human-AI Partnership Model
AI handles repetitive cross-referencing, while humans apply judgment and strategy. Compliance professionals can focus on novel questions and risk decisions, elevating their role from document reviewer to strategic advisor. Domain expertise remains essential in an AI-augmented workflow.
Building a Modern Validation Program
Evaluating Your Current State
Assess your validation process maturity by benchmarking review times, error rates, and identifying warning signs of overburdened manual processes. Are review cycles consistently delayed? Are errors or inconsistencies surfacing during audits or recalls? These are signals that modernization is needed.
Key Capabilities to Look For
Seek solutions with comprehensive regulatory coverage, transparent reasoning, audit trails, integration with existing workflows, and flexibility for multiple product categories. The right system should support both federal and state requirements and international standards for global brands.
Change Management Considerations
Secure buy-in from compliance teams by emphasizing augmentation, not replacement. Pilot programs and proof-of-concept approaches can demonstrate value, and ROI should be measured and communicated clearly. Change management is critical to ensure a smooth transition and lasting adoption.
The Future of Food Formula Validation
Regulatory complexity will continue to increase, and consumer expectations for transparency are rising. Supply chain traceability demands are expanding, making efficient, reliable compliance a competitive advantage. Forward-thinking organizations are positioning compliance as a strategic function rather than a cost center. Those who modernize their formula validation process will be best equipped to protect consumers, brands, and the broader supply chain.
Conclusion
Food formula validation is a cornerstone of food safety and compliance, protecting consumers, brands, and the broader supply chain. The dedication of compliance professionals ensures that every product meets rigorous standards, even as the landscape grows more complex. By embracing modern, AI-powered solutions, organizations can achieve greater efficiency and accuracy without sacrificing the rigor that underpins consumer trust. Compliance done well is not just a regulatory requirement—it is a vital safeguard for everyone involved.
Introduction
Behind every food product label lies a world of complexity. Food formula validation is the rigorous process that ensures every ingredient, claim, and nutritional value meets the highest standards of safety and regulatory compliance. For compliance professionals, this work is both a science and an art—balancing evolving regulations, consumer safety, and brand reputation.
The stakes could not be higher. A single misstep in the formula validation process can trigger regulatory action, product recalls, or even endanger public health. Yet, the professionals who carry out food formula validation often work behind the scenes, navigating a labyrinth of ingredient compliance, food safety verification, and formula review requirements.
This comprehensive guide will demystify the hidden complexity behind food formula validation. We will explore the manual processes that have long defined the industry, the expertise required to navigate them, and the far-reaching impact of validation failures. We will also look ahead to the future, where AI-powered compliance solutions are transforming how brands bring safe, compliant products to market. Whether you are a regulatory affairs specialist, a quality manager, or a business leader, this guide will equip you to meet today’s challenges and prepare for tomorrow’s demands.
What Is Food Formula Validation?
Definition and Scope
Food formula validation is the systematic review of a food product’s formula to ensure it meets all regulatory requirements before reaching the market. This process encompasses ingredient legality, concentration limits, labeling accuracy, and the substantiation of marketing claims. Unlike quality control or safety testing, which focus on finished products, formula validation scrutinizes the composition and documentation of each product at the earliest stages.
The formula validation process is a cornerstone of food product compliance. It involves a detailed food product formula review, where each ingredient is assessed for compliance with federal, state, and international standards. Ingredient validation ensures that every component is legal, safe, and accurately represented.
The Regulatory Landscape
The regulatory environment for food formula validation is complex and ever-changing. In the United States, the Food and Drug Administration (FDA) governs food ingredients, additives, and labeling through Title 21 of the Code of Federal Regulations (CFR). State-level requirements, such as California’s Proposition 65, add further complexity, while products intended for international markets must comply with foreign standards and import restrictions.
Each product category—conventional foods, dietary supplements, beverages, and functional foods—has its own regulatory nuances. For example, dietary supplements are regulated under the Dietary Supplement Health and Education Act (DSHEA), while functional foods may face additional scrutiny regarding health claims.
What Gets Validated
The formula validation process covers several critical areas:
Ingredient identity and specifications: Ensuring each component is accurately described and meets regulatory definitions.
Concentration levels and daily intake calculations: Verifying that ingredient amounts are within legal and safe limits.
Allergen declarations: Confirming that all potential allergens are properly disclosed.
Nutrient content and nutrition facts accuracy: Ensuring that nutritional information is correct and substantiated.
Marketing claims and their regulatory basis: Validating that all claims are supported by scientific evidence and permitted by law.
Label compliance: Reviewing required statements, formatting, and placement to meet regulatory standards.
The Manual Formula Validation Process
The formula validation process begins with document collection, where the reviewer gathers all relevant specifications, certificates of analysis, and supplier documentation needed to evaluate the product. With materials in hand, the reviewer works through each ingredient individually, cross-referencing components against regulatory databases to confirm legality and compliance status. This ingredient-level review feeds directly into concentration calculations, where the reviewer verifies that levels fall within permitted ranges after accounting for serving sizes and daily intake limits.

Once the formula itself checks out, attention shifts to the label. Label reconciliation ensures the formula matches what's declared on packaging and that all required information appears correctly. Any marketing claims are then verified to confirm they're supported by regulatory guidance and scientific evidence. The process concludes with documentation and sign-off, creating the audit trails and approval records that demonstrate due diligence and support future audits or regulatory inquiries.
Tools and Resources Used in Manual Review
Compliance professionals rely on a range of resources, including:
FDA databases (GRAS notices, food additive status lists, EAFUS)
CFR references and guidance documents
Proprietary ingredient databases
Spreadsheets and document management systems
Institutional knowledge and reference libraries
Time and Resource Requirements
The time required for a formula review varies widely. Simple products may require only a few hours, while complex formulations can take days or even weeks. Advanced degrees in food science or regulatory affairs are often necessary, and review cycles may involve multiple rounds of revision and documentation. The burden of record-keeping and audit trail maintenance adds further complexity.
The Expertise Behind the Process
Formula validation demands specialized knowledge. Regulatory affairs professionals must stay current with evolving regulations, interpret complex guidance, and apply scientific judgment to novel ingredients and claims. Ongoing training and professional development are essential to maintain expertise and ensure compliance.
Risks of Manual Formula Validation
Human Error in Complex Reviews
Manual reviews are susceptible to human error, especially when cross-referencing multiple regulations. Version control issues, transcription mistakes, and calculation errors can all compromise the integrity of the validation process. High-volume environments increase the risk of fatigue-related mistakes.
Knowledge Gaps and Regulatory Blind Spots
Keeping up with regulatory changes is a constant challenge. State-level requirements and international regulations may be overlooked, and novel ingredients can present ambiguous compliance questions.
Inconsistency Across Reviewers
Different reviewers may interpret regulations differently, leading to inconsistent outcomes. Lack of standardized processes and reliance on institutional knowledge can result in gaps when experienced staff leave the organization.
Documentation and Audit Trail Gaps
Incomplete records of review rationale make it difficult to demonstrate due diligence. Reconstructing review history during audits or recalls can be challenging, exposing organizations to regulatory risk.
Real-World Consequences of Validation Failures
Validation failures can lead to warning letters, enforcement actions, product recalls, and litigation. Recent FDA warning letters highlight the serious consequences of non-compliance, including financial losses and reputational damage. For example, in 2023, the FDA issued multiple warning letters to food manufacturers for mislabeling and undeclared allergens, resulting in costly recalls and heightened scrutiny.
The True Cost of Manual Validation

Direct Operating Costs
Manual validation requires a significant investment in specialized staff, resulting in high salaries and overhead costs. Extended review cycles can delay time-to-market, and external consultants may be needed for complex formulas.
Indirect and Hidden Costs
Opportunity costs arise when skilled professionals are tied up in manual reviews rather than strategic initiatives. Training and onboarding expenses, technology subscriptions, and the cost of rework when errors are discovered late in development all add to the total cost.
Scalability Challenges
As product portfolios grow, costs multiply. Seasonal launches and capacity crunches strain resources, and simply adding headcount yields diminishing returns without process improvements.
Stakeholder Impact: How Validation Failures Ripple Across the Supply Chain
Impact on Brands
Regulatory enforcement, warning letters, and recalls can damage brand reputation and strain retailer relationships. Consumer trust is eroded, and financial exposure from recalls and litigation can be significant. A single validation failure can have lasting consequences, as seen in high-profile industry cases.
Example: In 2022, a major snack brand faced a nationwide recall due to undeclared allergens, resulting in millions of dollars in lost sales and a significant drop in consumer trust.
Impact on Manufacturers
Production delays, waste from non-compliant batches, and damaged relationships with brand partners are common outcomes. Manufacturers may also face liability exposure and complications during audits and certifications.
Impact on Contract Manufacturing Partners
Co-packers and third-party manufacturers often have limited visibility into formula development decisions but share liability for compliance failures. Managing validation across multiple clients increases complexity and documentation burdens.
Impact on Retailers
Retailers face shelf liability for non-compliant products, regulatory scrutiny, and supply chain disruptions when products are pulled. Private label products present unique validation challenges, and consumer complaints must be managed effectively.
Impact on Consumers
Consumers are exposed to health and safety risks from undeclared allergens or unsafe ingredients. Misleading claims can affect purchasing decisions and health outcomes, particularly for vulnerable populations such as those with allergies, dietary restrictions, or children.
Modernizing Formula Validation with AI
The Case for Technology-Enabled Validation
The volume and complexity of modern food portfolios have outpaced manual validation capacity. Regulatory changes are accelerating, and skilled compliance professionals are in short supply. AI-powered solutions offer the opportunity to elevate human expertise by automating repetitive tasks and enabling strategic focus.
How AI Transforms the Validation Process
AI systems can automatically cross-reference formulas against comprehensive regulatory databases, flagging potential compliance issues in real time. They apply consistent regulatory logic across all reviews, instantly identify state-level and international requirements, and continuously monitor for regulatory changes.
Business Value of AI-Powered Validation
Speed: Review cycles are reduced from days to minutes.
Accuracy: Manual lookups and calculation errors are eliminated.
Consistency: Rigorous standards are applied to every formula.
Scalability: Portfolio growth is managed without proportional increases in headcount.
Auditability: Review logic and decisions are automatically documented.
Strategic reallocation: Compliance professionals are freed for higher-value work.
The Human-AI Partnership Model
AI handles repetitive cross-referencing, while humans apply judgment and strategy. Compliance professionals can focus on novel questions and risk decisions, elevating their role from document reviewer to strategic advisor. Domain expertise remains essential in an AI-augmented workflow.
Building a Modern Validation Program
Evaluating Your Current State
Assess your validation process maturity by benchmarking review times, error rates, and identifying warning signs of overburdened manual processes. Are review cycles consistently delayed? Are errors or inconsistencies surfacing during audits or recalls? These are signals that modernization is needed.
Key Capabilities to Look For
Seek solutions with comprehensive regulatory coverage, transparent reasoning, audit trails, integration with existing workflows, and flexibility for multiple product categories. The right system should support both federal and state requirements and international standards for global brands.
Change Management Considerations
Secure buy-in from compliance teams by emphasizing augmentation, not replacement. Pilot programs and proof-of-concept approaches can demonstrate value, and ROI should be measured and communicated clearly. Change management is critical to ensure a smooth transition and lasting adoption.
The Future of Food Formula Validation
Regulatory complexity will continue to increase, and consumer expectations for transparency are rising. Supply chain traceability demands are expanding, making efficient, reliable compliance a competitive advantage. Forward-thinking organizations are positioning compliance as a strategic function rather than a cost center. Those who modernize their formula validation process will be best equipped to protect consumers, brands, and the broader supply chain.
Conclusion
Food formula validation is a cornerstone of food safety and compliance, protecting consumers, brands, and the broader supply chain. The dedication of compliance professionals ensures that every product meets rigorous standards, even as the landscape grows more complex. By embracing modern, AI-powered solutions, organizations can achieve greater efficiency and accuracy without sacrificing the rigor that underpins consumer trust. Compliance done well is not just a regulatory requirement—it is a vital safeguard for everyone involved.
The information presented is for educational and informational purposes only and should not be construed as legal, regulatory, or professional advice. Organizations should consult with qualified legal and compliance professionals for guidance specific to their circumstances.
Why Food Formula Validation Matters: Insights for Food Manufacturers
Why Food Formula Validation Matters: Insights for Food Manufacturers
Jan 21, 2026