Jan 22, 2026
Articles
Jan 22, 2026
Articles
Jan 22, 2026
Articles
The Complete Guide to Dietary Supplements Formula Validation: Process, Risks, and Modern Solutions

Martín Ramírez

Martín Ramírez

Martín Ramírez

Dietary supplement teams move fast: new ingredients, new suppliers, refreshed claims, seasonal launches, retailer-specific packs. Speed is a competitive advantage, yet it quietly increases a different kind of exposure: formula and label mistakes that slip past busy reviewers.
That is why dietary supplements formula validation has become a defining discipline for compliance professionals, quality leaders, and operations owners. When done well, it creates a repeatable way to confirm that a product’s formulation, specs, and label all match and comply with the rules of the markets where the product will be sold.
When done inconsistently, the costs show up everywhere: change controls that never close, avoidable deviations, reformulations that could have been prevented, delayed POs, retailer chargebacks, and the worst case, consumer harm. The good news is that the process can be made both stricter and faster, especially when modern systems reduce manual checking and improve traceability.
What dietary supplements formula validation actually is
Dietary supplements formula validation is the structured verification that a product’s formula, specs, manufacturing intent, and label agree with each other and satisfy applicable requirements. It is not only a “label check,” and it is not only an R&D signoff. It sits at the intersection of product design, quality systems, and dietary supplement regulatory compliance.
At a practical level, formula validation answers questions like: Are ingredient identities and forms consistent across documents? Do declared amounts match the bill of materials and overage strategy? Do excipients align with processing aids and allergen statements? Are warnings, directions, and claims supported and permitted for the target channel and region?
It also supports supplement compliance by turning ambiguous assumptions into documented decisions. Many teams rely on tacit knowledge: “We always do it this way,” or “QA knows the limits.” Formula validation makes those assumptions visible and testable.
A useful way to think about it is as a reconciliation exercise across three layers:
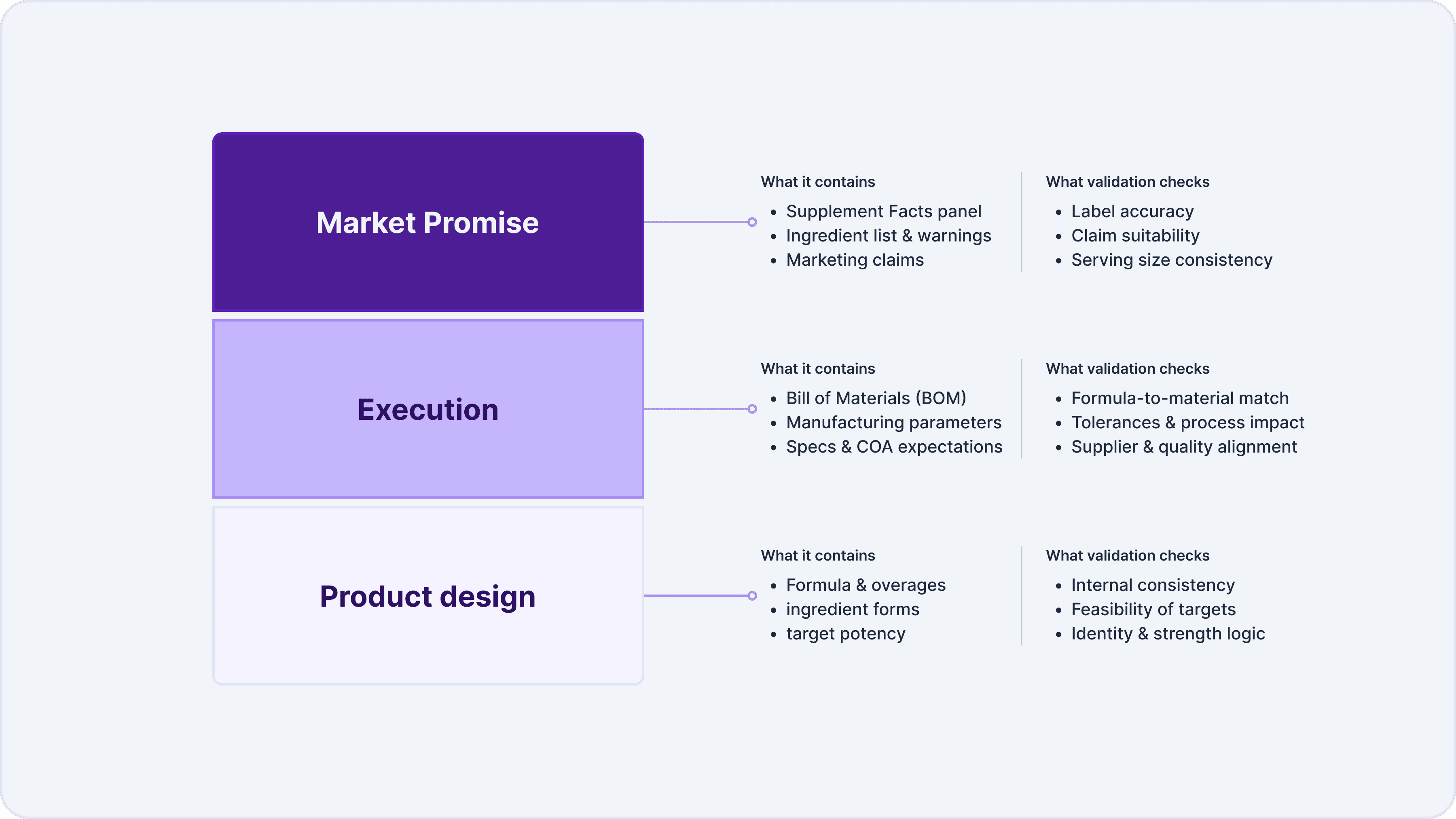
Strong programs treat formula validation as a lifecycle activity. The first review occurs before launch, and it repeats whenever a supplier changes, an ingredient spec changes, an overage strategy is adjusted, a label is refreshed, or a product expands into a new channel.
The manual formula review process most teams inherit
Manual review is common because it starts simply: a few documents, a shared drive, and experienced reviewers. Over time, complexity grows. Product families multiply, suppliers diversify, and regulatory expectations rise, while the workflow still depends on copy-paste and human memory.
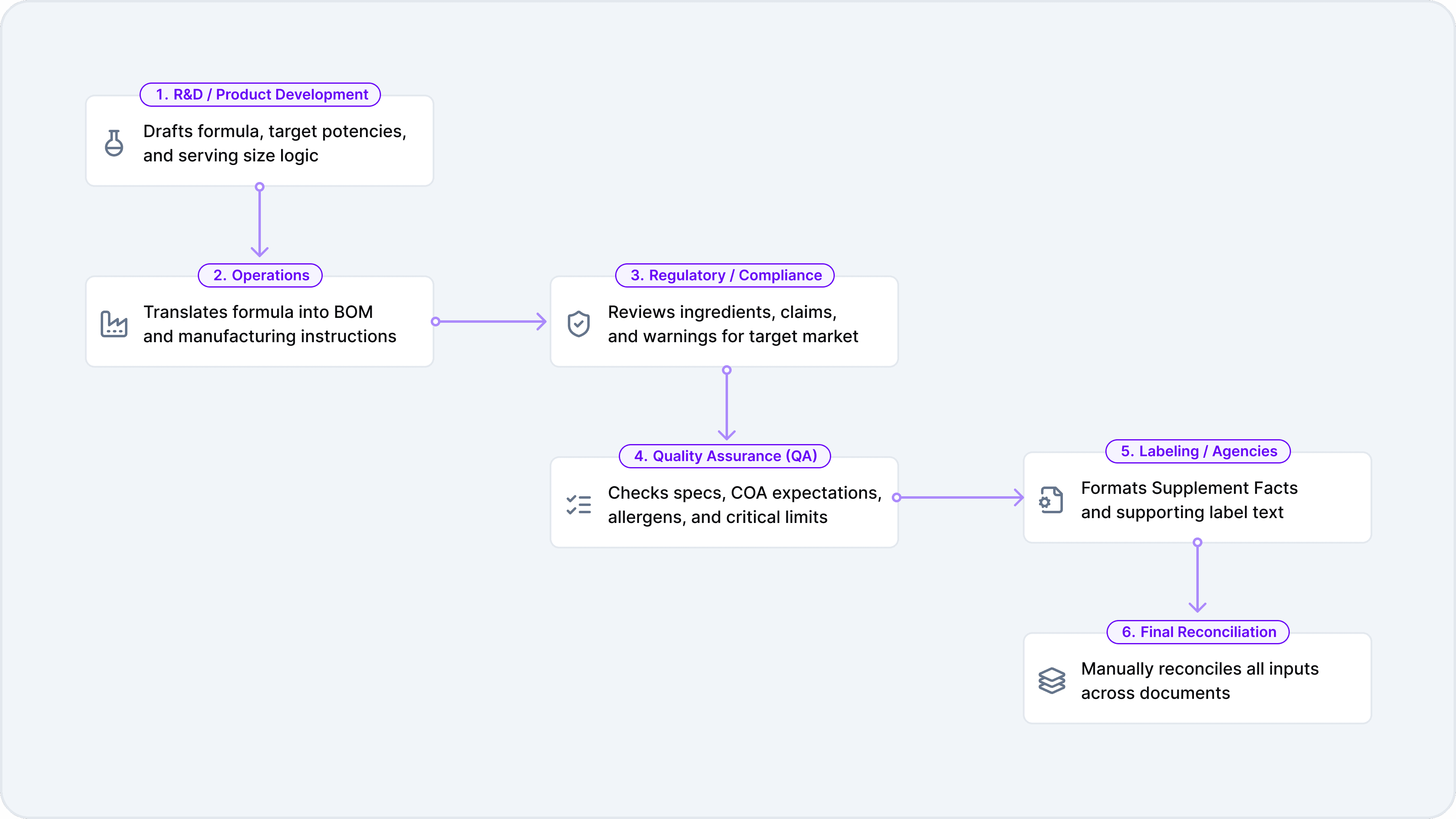
A typical formula review process touches several systems and handoffs, often under tight timelines. It begins when R&D or product development drafts the formula, establishing target potencies and serving size logic. From there, operations translates that formula into a bill of materials and manufacturing instructions that can actually be produced at scale. Regulatory or compliance teams then review the ingredients, claims, and required warnings for each intended market, while QA checks specifications, COA expectations, allergens, and critical limits.
Once those reviews are complete, labeling teams or external agencies format the Supplement Facts panel and supporting text to meet FDA requirements. Finally, someone—often armed with spreadsheets and annotated PDFs—reconciles everything to ensure the development formula, manufacturing BOM, quality specifications, and label artwork all align before the product can move forward.
After that, change control begins its own loop: updates trigger re-review, which triggers new label versions, which trigger new approvals, which trigger rework in the BOM or spec files.
Common manual artifacts include spreadsheet calculators for label math, checklists for required statements, email approval chains, and “final-final-v7” label files. These tools can work, yet they do not age well when portfolios and partner networks scale.
The biggest limitation is not effort. It is that the manual process struggles to prove what happened, when it happened, and why a decision was made.
After teams have mapped their process, it helps to name the checkpoints that must be true every time:
Draft formula completed
BOM and batch record created
Claims and warnings reviewed
Specs and allergens verified
Label text and panel math reconciled
Where manual validation goes wrong
Manual validation fails in predictable ways, even with talented reviewers. The failures usually come from fragmentation: data lives in multiple places, and each handoff introduces translation risk.
One failure mode is mismatched ingredient identity. A label might say “magnesium (as glycinate)” while purchasing buys magnesium bisglycinate chelate with a different elemental yield. The formula is “right” in one document and “right” in another, yet the product promise is not aligned.
Another is math drift. Serving size changes, capsule count changes, or overages are adjusted for stability, and the label calculator is not updated everywhere. The Supplement Facts panel remains internally consistent, yet it no longer matches the executed BOM.
A third is unclear assumptions. Teams may interpret “extract” differently, apply different rounding rules, or rely on informal limits for certain ingredients. When assumptions are not recorded, reviewers cannot reliably reproduce the decision later, especially during audits or retailer inquiries.
Manual methods also struggle with the edges of compliance: markets with different required statements, channel-specific restrictions on claims, and the nuance of what must be on-pack versus in supporting materials. That is where dietary supplement regulatory compliance shifts from memorizing rules to managing versions, evidence, and traceability.
The most common root causes tend to cluster into a small set:
Single point reviewers: one expert becomes the workflow bottleneck
Document drift: PDFs, spreadsheets, and PLM fields disagree over time
Weak change control: edits occur outside the formal system
Inconsistent rule application: different reviewers apply different thresholds
Late-stage surprises: issues found after print-ready artwork
Impact on brands: speed, credibility, and cost control
Brands live at the intersection of innovation and scrutiny. When formula validation is slow or inconsistent, launches slip, and teams compensate by compressing review windows, which increases risk.
The operational cost shows up in rework. A label correction might require artwork revision, print scrap, new retailer submissions, and delayed revenue. Even when issues are caught before shipment, the opportunity cost is real: the team spends time fixing preventable mismatches instead of building the next product.
Credibility is the quieter impact. When brands cannot quickly answer questions about formula intent, overage rationale, or label math, partners lose confidence. Retailers and platforms increasingly expect prompt, well-organized responses during onboarding and periodic reviews. Strong supplement compliance practices make those responses routine, not stressful.
Brands also face a compounding effect: each SKU variation multiplies review volume. One formula with five pack sizes and three channels can create a web of versions. Without disciplined validation, that web becomes fragile.
Impact on manufacturers and contract manufacturing partners
For manufacturers, the pain is often more immediate: deviations, holds, rework, and line downtime. A small documentation mismatch can halt production while teams clarify whether the label, BOM, and batch record are aligned.
Contract manufacturers also operate across many brand-specific rules. One customer may accept certain overage practices; another may require a different approach to rounding or label presentation. If expectations are not captured clearly during validation, the manufacturer absorbs the churn during execution.
Quality teams feel the pressure during investigations. When a complaint or out-of-spec result arrives, the team needs a clean lineage: which ingredient lots were used, which COA limits applied, which label version was active, and what the intended potency was at release and through shelf life.
A modern validation discipline can reduce friction between partners by clarifying “what good looks like” before production begins and producing artifacts that can be shared without ambiguity.
Impact on retailers: intake efficiency and brand risk
Retailers have become de facto gatekeepers. Their teams review labels, claims, allergens, certificates, and product data at scale. When submissions contain inconsistencies, the retailer experience becomes slower for everyone: more questions, more resubmissions, more queue time.
Retailers also carry reputational risk. A product that generates consumer complaints or regulatory scrutiny reflects not only on the brand but also on the shelf. That reality is driving more rigorous intake standards and ongoing monitoring.
For brands selling through multiple retailers, formula validation becomes the foundation for predictable retail execution. It supports supplement label verification across channels, with fewer surprises when a retailer applies a stricter interpretation than a brand expected.
The highest stakes: consumer safety
Consumer safety is not a slogan in this space. It is the practical outcome of getting identity, strength, allergens, directions, and warnings right, every time.
A misdeclared allergen, a potency mismatch, or confusing directions can create real harm. Even when the product is manufactured correctly, a label error can still mislead. Conversely, even with a perfect label, a formula-document mismatch can cause the manufacturer to produce something different from what the consumer expects.
Safety risk is also a trust risk. When consumers lose confidence in supplement quality, the entire category pays the price through increased scrutiny and reduced willingness to try new products.
A well-run validation process acts as a safety net that catches issues upstream, when fixes are cheap and fast.
How AI modernizes dietary supplements formula validation
AI is most valuable here when it reduces manual comparison work and raises the consistency of rule application. The goal is not to replace regulatory judgment. The goal is to give reviewers cleaner inputs, fewer blind spots, and stronger traceability.
At a workflow level, AI can help in four ways:
1) Document normalization and extraction. AI can read common inputs such as specs, COAs, formulas, and label proofs, and structure key fields: ingredient names, forms, units, limits, serving size, allergen statements, and claims. That reduces copy-paste errors and makes cross-document checks practical.
2) Automated reconciliation. Once data is structured, systems can compare the formula to the BOM, the BOM to the batch record, and all of it to the label. Discrepancies can be flagged early: unit mismatches, rounding differences, ingredient form inconsistencies, and missing statements.
3) Rule-aware review support. With a curated rule set, AI can highlight risk areas tied to the intended market and channel. This is where dietary supplement regulatory compliance becomes operational: the system prompts reviewers to confirm required elements and records the decision path.
4) Evidence and audit trails. AI-supported workflows can automatically store the “why” behind approvals, including which documents were reviewed, what version, what differences were found, and how they were resolved. That is valuable during internal audits, retailer questions, and regulatory inquiries.
The strongest implementations focus on decision quality and accountability:
Consistency: the same rule checks every SKU, every time
Transparency: reviewers can see what triggered a flag and what data it used
Control: human approvers accept, reject, or override with documented rationale
AI also changes capacity planning. Instead of scaling by adding reviewers, teams can scale by standardizing inputs and letting automation handle the repetitive comparisons that consume expert time.
Building a modern formula validation practice
Modernization does not require a full system replacement. It requires a clear operating model and a commitment to data discipline.
Start by defining the authoritative source for each element: ingredient identity, label amounts, allergen statements, claim language, and specs. Then define what constitutes a required validation event, including supplier changes and any label updates.
It also helps to assign explicit ownership so validation does not float between departments. A lightweight RACI and a single validation queue can prevent last-minute scrambles.
A practical set of design principles can guide implementation:
Single source of truth: one governed record for formula and label-critical fields
Change-triggered validation: reviews automatically restart when key inputs change
Closed-loop discrepancy handling: each flag must be resolved, not ignored
Over time, teams can measure maturity through a few metrics: discrepancy rate per SKU, cycle time from draft to approved label, percent of changes routed through change control, and the share of validations supported by structured data rather than manual transcription.
Progress here tends to be contagious. When compliance, QA, and operations can trust the same validated dataset, launches become calmer, partner interactions become smoother, and teams regain time for higher-value work.
Dietary supplement teams move fast: new ingredients, new suppliers, refreshed claims, seasonal launches, retailer-specific packs. Speed is a competitive advantage, yet it quietly increases a different kind of exposure: formula and label mistakes that slip past busy reviewers.
That is why dietary supplements formula validation has become a defining discipline for compliance professionals, quality leaders, and operations owners. When done well, it creates a repeatable way to confirm that a product’s formulation, specs, and label all match and comply with the rules of the markets where the product will be sold.
When done inconsistently, the costs show up everywhere: change controls that never close, avoidable deviations, reformulations that could have been prevented, delayed POs, retailer chargebacks, and the worst case, consumer harm. The good news is that the process can be made both stricter and faster, especially when modern systems reduce manual checking and improve traceability.
What dietary supplements formula validation actually is
Dietary supplements formula validation is the structured verification that a product’s formula, specs, manufacturing intent, and label agree with each other and satisfy applicable requirements. It is not only a “label check,” and it is not only an R&D signoff. It sits at the intersection of product design, quality systems, and dietary supplement regulatory compliance.
At a practical level, formula validation answers questions like: Are ingredient identities and forms consistent across documents? Do declared amounts match the bill of materials and overage strategy? Do excipients align with processing aids and allergen statements? Are warnings, directions, and claims supported and permitted for the target channel and region?
It also supports supplement compliance by turning ambiguous assumptions into documented decisions. Many teams rely on tacit knowledge: “We always do it this way,” or “QA knows the limits.” Formula validation makes those assumptions visible and testable.
A useful way to think about it is as a reconciliation exercise across three layers:

Strong programs treat formula validation as a lifecycle activity. The first review occurs before launch, and it repeats whenever a supplier changes, an ingredient spec changes, an overage strategy is adjusted, a label is refreshed, or a product expands into a new channel.
The manual formula review process most teams inherit
Manual review is common because it starts simply: a few documents, a shared drive, and experienced reviewers. Over time, complexity grows. Product families multiply, suppliers diversify, and regulatory expectations rise, while the workflow still depends on copy-paste and human memory.

A typical formula review process touches several systems and handoffs, often under tight timelines. It begins when R&D or product development drafts the formula, establishing target potencies and serving size logic. From there, operations translates that formula into a bill of materials and manufacturing instructions that can actually be produced at scale. Regulatory or compliance teams then review the ingredients, claims, and required warnings for each intended market, while QA checks specifications, COA expectations, allergens, and critical limits.
Once those reviews are complete, labeling teams or external agencies format the Supplement Facts panel and supporting text to meet FDA requirements. Finally, someone—often armed with spreadsheets and annotated PDFs—reconciles everything to ensure the development formula, manufacturing BOM, quality specifications, and label artwork all align before the product can move forward.
After that, change control begins its own loop: updates trigger re-review, which triggers new label versions, which trigger new approvals, which trigger rework in the BOM or spec files.
Common manual artifacts include spreadsheet calculators for label math, checklists for required statements, email approval chains, and “final-final-v7” label files. These tools can work, yet they do not age well when portfolios and partner networks scale.
The biggest limitation is not effort. It is that the manual process struggles to prove what happened, when it happened, and why a decision was made.
After teams have mapped their process, it helps to name the checkpoints that must be true every time:
Draft formula completed
BOM and batch record created
Claims and warnings reviewed
Specs and allergens verified
Label text and panel math reconciled
Where manual validation goes wrong
Manual validation fails in predictable ways, even with talented reviewers. The failures usually come from fragmentation: data lives in multiple places, and each handoff introduces translation risk.
One failure mode is mismatched ingredient identity. A label might say “magnesium (as glycinate)” while purchasing buys magnesium bisglycinate chelate with a different elemental yield. The formula is “right” in one document and “right” in another, yet the product promise is not aligned.
Another is math drift. Serving size changes, capsule count changes, or overages are adjusted for stability, and the label calculator is not updated everywhere. The Supplement Facts panel remains internally consistent, yet it no longer matches the executed BOM.
A third is unclear assumptions. Teams may interpret “extract” differently, apply different rounding rules, or rely on informal limits for certain ingredients. When assumptions are not recorded, reviewers cannot reliably reproduce the decision later, especially during audits or retailer inquiries.
Manual methods also struggle with the edges of compliance: markets with different required statements, channel-specific restrictions on claims, and the nuance of what must be on-pack versus in supporting materials. That is where dietary supplement regulatory compliance shifts from memorizing rules to managing versions, evidence, and traceability.
The most common root causes tend to cluster into a small set:
Single point reviewers: one expert becomes the workflow bottleneck
Document drift: PDFs, spreadsheets, and PLM fields disagree over time
Weak change control: edits occur outside the formal system
Inconsistent rule application: different reviewers apply different thresholds
Late-stage surprises: issues found after print-ready artwork
Impact on brands: speed, credibility, and cost control
Brands live at the intersection of innovation and scrutiny. When formula validation is slow or inconsistent, launches slip, and teams compensate by compressing review windows, which increases risk.
The operational cost shows up in rework. A label correction might require artwork revision, print scrap, new retailer submissions, and delayed revenue. Even when issues are caught before shipment, the opportunity cost is real: the team spends time fixing preventable mismatches instead of building the next product.
Credibility is the quieter impact. When brands cannot quickly answer questions about formula intent, overage rationale, or label math, partners lose confidence. Retailers and platforms increasingly expect prompt, well-organized responses during onboarding and periodic reviews. Strong supplement compliance practices make those responses routine, not stressful.
Brands also face a compounding effect: each SKU variation multiplies review volume. One formula with five pack sizes and three channels can create a web of versions. Without disciplined validation, that web becomes fragile.
Impact on manufacturers and contract manufacturing partners
For manufacturers, the pain is often more immediate: deviations, holds, rework, and line downtime. A small documentation mismatch can halt production while teams clarify whether the label, BOM, and batch record are aligned.
Contract manufacturers also operate across many brand-specific rules. One customer may accept certain overage practices; another may require a different approach to rounding or label presentation. If expectations are not captured clearly during validation, the manufacturer absorbs the churn during execution.
Quality teams feel the pressure during investigations. When a complaint or out-of-spec result arrives, the team needs a clean lineage: which ingredient lots were used, which COA limits applied, which label version was active, and what the intended potency was at release and through shelf life.
A modern validation discipline can reduce friction between partners by clarifying “what good looks like” before production begins and producing artifacts that can be shared without ambiguity.
Impact on retailers: intake efficiency and brand risk
Retailers have become de facto gatekeepers. Their teams review labels, claims, allergens, certificates, and product data at scale. When submissions contain inconsistencies, the retailer experience becomes slower for everyone: more questions, more resubmissions, more queue time.
Retailers also carry reputational risk. A product that generates consumer complaints or regulatory scrutiny reflects not only on the brand but also on the shelf. That reality is driving more rigorous intake standards and ongoing monitoring.
For brands selling through multiple retailers, formula validation becomes the foundation for predictable retail execution. It supports supplement label verification across channels, with fewer surprises when a retailer applies a stricter interpretation than a brand expected.
The highest stakes: consumer safety
Consumer safety is not a slogan in this space. It is the practical outcome of getting identity, strength, allergens, directions, and warnings right, every time.
A misdeclared allergen, a potency mismatch, or confusing directions can create real harm. Even when the product is manufactured correctly, a label error can still mislead. Conversely, even with a perfect label, a formula-document mismatch can cause the manufacturer to produce something different from what the consumer expects.
Safety risk is also a trust risk. When consumers lose confidence in supplement quality, the entire category pays the price through increased scrutiny and reduced willingness to try new products.
A well-run validation process acts as a safety net that catches issues upstream, when fixes are cheap and fast.
How AI modernizes dietary supplements formula validation
AI is most valuable here when it reduces manual comparison work and raises the consistency of rule application. The goal is not to replace regulatory judgment. The goal is to give reviewers cleaner inputs, fewer blind spots, and stronger traceability.
At a workflow level, AI can help in four ways:
1) Document normalization and extraction. AI can read common inputs such as specs, COAs, formulas, and label proofs, and structure key fields: ingredient names, forms, units, limits, serving size, allergen statements, and claims. That reduces copy-paste errors and makes cross-document checks practical.
2) Automated reconciliation. Once data is structured, systems can compare the formula to the BOM, the BOM to the batch record, and all of it to the label. Discrepancies can be flagged early: unit mismatches, rounding differences, ingredient form inconsistencies, and missing statements.
3) Rule-aware review support. With a curated rule set, AI can highlight risk areas tied to the intended market and channel. This is where dietary supplement regulatory compliance becomes operational: the system prompts reviewers to confirm required elements and records the decision path.
4) Evidence and audit trails. AI-supported workflows can automatically store the “why” behind approvals, including which documents were reviewed, what version, what differences were found, and how they were resolved. That is valuable during internal audits, retailer questions, and regulatory inquiries.
The strongest implementations focus on decision quality and accountability:
Consistency: the same rule checks every SKU, every time
Transparency: reviewers can see what triggered a flag and what data it used
Control: human approvers accept, reject, or override with documented rationale
AI also changes capacity planning. Instead of scaling by adding reviewers, teams can scale by standardizing inputs and letting automation handle the repetitive comparisons that consume expert time.
Building a modern formula validation practice
Modernization does not require a full system replacement. It requires a clear operating model and a commitment to data discipline.
Start by defining the authoritative source for each element: ingredient identity, label amounts, allergen statements, claim language, and specs. Then define what constitutes a required validation event, including supplier changes and any label updates.
It also helps to assign explicit ownership so validation does not float between departments. A lightweight RACI and a single validation queue can prevent last-minute scrambles.
A practical set of design principles can guide implementation:
Single source of truth: one governed record for formula and label-critical fields
Change-triggered validation: reviews automatically restart when key inputs change
Closed-loop discrepancy handling: each flag must be resolved, not ignored
Over time, teams can measure maturity through a few metrics: discrepancy rate per SKU, cycle time from draft to approved label, percent of changes routed through change control, and the share of validations supported by structured data rather than manual transcription.
Progress here tends to be contagious. When compliance, QA, and operations can trust the same validated dataset, launches become calmer, partner interactions become smoother, and teams regain time for higher-value work.
Dietary supplement teams move fast: new ingredients, new suppliers, refreshed claims, seasonal launches, retailer-specific packs. Speed is a competitive advantage, yet it quietly increases a different kind of exposure: formula and label mistakes that slip past busy reviewers.
That is why dietary supplements formula validation has become a defining discipline for compliance professionals, quality leaders, and operations owners. When done well, it creates a repeatable way to confirm that a product’s formulation, specs, and label all match and comply with the rules of the markets where the product will be sold.
When done inconsistently, the costs show up everywhere: change controls that never close, avoidable deviations, reformulations that could have been prevented, delayed POs, retailer chargebacks, and the worst case, consumer harm. The good news is that the process can be made both stricter and faster, especially when modern systems reduce manual checking and improve traceability.
What dietary supplements formula validation actually is
Dietary supplements formula validation is the structured verification that a product’s formula, specs, manufacturing intent, and label agree with each other and satisfy applicable requirements. It is not only a “label check,” and it is not only an R&D signoff. It sits at the intersection of product design, quality systems, and dietary supplement regulatory compliance.
At a practical level, formula validation answers questions like: Are ingredient identities and forms consistent across documents? Do declared amounts match the bill of materials and overage strategy? Do excipients align with processing aids and allergen statements? Are warnings, directions, and claims supported and permitted for the target channel and region?
It also supports supplement compliance by turning ambiguous assumptions into documented decisions. Many teams rely on tacit knowledge: “We always do it this way,” or “QA knows the limits.” Formula validation makes those assumptions visible and testable.
A useful way to think about it is as a reconciliation exercise across three layers:

Strong programs treat formula validation as a lifecycle activity. The first review occurs before launch, and it repeats whenever a supplier changes, an ingredient spec changes, an overage strategy is adjusted, a label is refreshed, or a product expands into a new channel.
The manual formula review process most teams inherit
Manual review is common because it starts simply: a few documents, a shared drive, and experienced reviewers. Over time, complexity grows. Product families multiply, suppliers diversify, and regulatory expectations rise, while the workflow still depends on copy-paste and human memory.

A typical formula review process touches several systems and handoffs, often under tight timelines. It begins when R&D or product development drafts the formula, establishing target potencies and serving size logic. From there, operations translates that formula into a bill of materials and manufacturing instructions that can actually be produced at scale. Regulatory or compliance teams then review the ingredients, claims, and required warnings for each intended market, while QA checks specifications, COA expectations, allergens, and critical limits.
Once those reviews are complete, labeling teams or external agencies format the Supplement Facts panel and supporting text to meet FDA requirements. Finally, someone—often armed with spreadsheets and annotated PDFs—reconciles everything to ensure the development formula, manufacturing BOM, quality specifications, and label artwork all align before the product can move forward.
After that, change control begins its own loop: updates trigger re-review, which triggers new label versions, which trigger new approvals, which trigger rework in the BOM or spec files.
Common manual artifacts include spreadsheet calculators for label math, checklists for required statements, email approval chains, and “final-final-v7” label files. These tools can work, yet they do not age well when portfolios and partner networks scale.
The biggest limitation is not effort. It is that the manual process struggles to prove what happened, when it happened, and why a decision was made.
After teams have mapped their process, it helps to name the checkpoints that must be true every time:
Draft formula completed
BOM and batch record created
Claims and warnings reviewed
Specs and allergens verified
Label text and panel math reconciled
Where manual validation goes wrong
Manual validation fails in predictable ways, even with talented reviewers. The failures usually come from fragmentation: data lives in multiple places, and each handoff introduces translation risk.
One failure mode is mismatched ingredient identity. A label might say “magnesium (as glycinate)” while purchasing buys magnesium bisglycinate chelate with a different elemental yield. The formula is “right” in one document and “right” in another, yet the product promise is not aligned.
Another is math drift. Serving size changes, capsule count changes, or overages are adjusted for stability, and the label calculator is not updated everywhere. The Supplement Facts panel remains internally consistent, yet it no longer matches the executed BOM.
A third is unclear assumptions. Teams may interpret “extract” differently, apply different rounding rules, or rely on informal limits for certain ingredients. When assumptions are not recorded, reviewers cannot reliably reproduce the decision later, especially during audits or retailer inquiries.
Manual methods also struggle with the edges of compliance: markets with different required statements, channel-specific restrictions on claims, and the nuance of what must be on-pack versus in supporting materials. That is where dietary supplement regulatory compliance shifts from memorizing rules to managing versions, evidence, and traceability.
The most common root causes tend to cluster into a small set:
Single point reviewers: one expert becomes the workflow bottleneck
Document drift: PDFs, spreadsheets, and PLM fields disagree over time
Weak change control: edits occur outside the formal system
Inconsistent rule application: different reviewers apply different thresholds
Late-stage surprises: issues found after print-ready artwork
Impact on brands: speed, credibility, and cost control
Brands live at the intersection of innovation and scrutiny. When formula validation is slow or inconsistent, launches slip, and teams compensate by compressing review windows, which increases risk.
The operational cost shows up in rework. A label correction might require artwork revision, print scrap, new retailer submissions, and delayed revenue. Even when issues are caught before shipment, the opportunity cost is real: the team spends time fixing preventable mismatches instead of building the next product.
Credibility is the quieter impact. When brands cannot quickly answer questions about formula intent, overage rationale, or label math, partners lose confidence. Retailers and platforms increasingly expect prompt, well-organized responses during onboarding and periodic reviews. Strong supplement compliance practices make those responses routine, not stressful.
Brands also face a compounding effect: each SKU variation multiplies review volume. One formula with five pack sizes and three channels can create a web of versions. Without disciplined validation, that web becomes fragile.
Impact on manufacturers and contract manufacturing partners
For manufacturers, the pain is often more immediate: deviations, holds, rework, and line downtime. A small documentation mismatch can halt production while teams clarify whether the label, BOM, and batch record are aligned.
Contract manufacturers also operate across many brand-specific rules. One customer may accept certain overage practices; another may require a different approach to rounding or label presentation. If expectations are not captured clearly during validation, the manufacturer absorbs the churn during execution.
Quality teams feel the pressure during investigations. When a complaint or out-of-spec result arrives, the team needs a clean lineage: which ingredient lots were used, which COA limits applied, which label version was active, and what the intended potency was at release and through shelf life.
A modern validation discipline can reduce friction between partners by clarifying “what good looks like” before production begins and producing artifacts that can be shared without ambiguity.
Impact on retailers: intake efficiency and brand risk
Retailers have become de facto gatekeepers. Their teams review labels, claims, allergens, certificates, and product data at scale. When submissions contain inconsistencies, the retailer experience becomes slower for everyone: more questions, more resubmissions, more queue time.
Retailers also carry reputational risk. A product that generates consumer complaints or regulatory scrutiny reflects not only on the brand but also on the shelf. That reality is driving more rigorous intake standards and ongoing monitoring.
For brands selling through multiple retailers, formula validation becomes the foundation for predictable retail execution. It supports supplement label verification across channels, with fewer surprises when a retailer applies a stricter interpretation than a brand expected.
The highest stakes: consumer safety
Consumer safety is not a slogan in this space. It is the practical outcome of getting identity, strength, allergens, directions, and warnings right, every time.
A misdeclared allergen, a potency mismatch, or confusing directions can create real harm. Even when the product is manufactured correctly, a label error can still mislead. Conversely, even with a perfect label, a formula-document mismatch can cause the manufacturer to produce something different from what the consumer expects.
Safety risk is also a trust risk. When consumers lose confidence in supplement quality, the entire category pays the price through increased scrutiny and reduced willingness to try new products.
A well-run validation process acts as a safety net that catches issues upstream, when fixes are cheap and fast.
How AI modernizes dietary supplements formula validation
AI is most valuable here when it reduces manual comparison work and raises the consistency of rule application. The goal is not to replace regulatory judgment. The goal is to give reviewers cleaner inputs, fewer blind spots, and stronger traceability.
At a workflow level, AI can help in four ways:
1) Document normalization and extraction. AI can read common inputs such as specs, COAs, formulas, and label proofs, and structure key fields: ingredient names, forms, units, limits, serving size, allergen statements, and claims. That reduces copy-paste errors and makes cross-document checks practical.
2) Automated reconciliation. Once data is structured, systems can compare the formula to the BOM, the BOM to the batch record, and all of it to the label. Discrepancies can be flagged early: unit mismatches, rounding differences, ingredient form inconsistencies, and missing statements.
3) Rule-aware review support. With a curated rule set, AI can highlight risk areas tied to the intended market and channel. This is where dietary supplement regulatory compliance becomes operational: the system prompts reviewers to confirm required elements and records the decision path.
4) Evidence and audit trails. AI-supported workflows can automatically store the “why” behind approvals, including which documents were reviewed, what version, what differences were found, and how they were resolved. That is valuable during internal audits, retailer questions, and regulatory inquiries.
The strongest implementations focus on decision quality and accountability:
Consistency: the same rule checks every SKU, every time
Transparency: reviewers can see what triggered a flag and what data it used
Control: human approvers accept, reject, or override with documented rationale
AI also changes capacity planning. Instead of scaling by adding reviewers, teams can scale by standardizing inputs and letting automation handle the repetitive comparisons that consume expert time.
Building a modern formula validation practice
Modernization does not require a full system replacement. It requires a clear operating model and a commitment to data discipline.
Start by defining the authoritative source for each element: ingredient identity, label amounts, allergen statements, claim language, and specs. Then define what constitutes a required validation event, including supplier changes and any label updates.
It also helps to assign explicit ownership so validation does not float between departments. A lightweight RACI and a single validation queue can prevent last-minute scrambles.
A practical set of design principles can guide implementation:
Single source of truth: one governed record for formula and label-critical fields
Change-triggered validation: reviews automatically restart when key inputs change
Closed-loop discrepancy handling: each flag must be resolved, not ignored
Over time, teams can measure maturity through a few metrics: discrepancy rate per SKU, cycle time from draft to approved label, percent of changes routed through change control, and the share of validations supported by structured data rather than manual transcription.
Progress here tends to be contagious. When compliance, QA, and operations can trust the same validated dataset, launches become calmer, partner interactions become smoother, and teams regain time for higher-value work.
The information presented is for educational and informational purposes only and should not be construed as legal, regulatory, or professional advice. Organizations should consult with qualified legal and compliance professionals for guidance specific to their circumstances.
The Complete Guide to Dietary Supplements Formula Validation: Process, Risks, and Modern Solutions
The Complete Guide to Dietary Supplements Formula Validation: Process, Risks, and Modern Solutions
Jan 22, 2026