Jun 18, 2025
Articles
Jun 18, 2025
Articles
Jun 18, 2025
Articles
What is the Food Safety Modernization Act (FSMA)? [Guide]

Martin Ramirez

Martin Ramirez

Martin Ramirez

The FDA’s enforcement of the Food Safety Modernization Act (FSMA) is designed to strengthen public health protections, prevent foodborne illness, and ensure traceability across the supply chain.
In this guide, we’ll break down the core Food Safety Modernization Act compliance areas, walk through key regulatory checks, and share practical tips for food businesses to meet FDA standards and avoid costly disruptions.
Let’s get started.
What is FSMA?
The Food Safety Modernization Act (FSMA) is a landmark U.S. law, signed by President Obama on January 4, 2011, aimed at fundamentally transforming the nation’s food safety system.
FSMA shifted the model from reactive (i.e., responding to outbreaks) to proactive (i.e., preventing them).
It gave the FDA sweeping new authorities, including mandatory recalls, risk-based standards, farm-to-fork traceability, and enhanced global oversight, enabling food safety grounded in science and prevention.
➸ At a Glance: Key Facts
Signing date: January 4, 2011
Primary aim: Prevention-first food safety coordinated across the entire food chain
Health imperative: Based on CDC estimates (~48 million illnesses, ~3,000 deaths per year)

FSMA's scope encompasses farms, manufacturers, transporters, and importers, requiring written preventive plans, stronger inspections, and thorough recordkeeping.
FSMA’s Core Goal: Prevention Over Reaction
Instead of waiting for contamination to cause outbreaks, FSMA requires businesses to:
Identify hazards early in the supply chain to prevent potential issues.
Implement preventive controls (e.g., kill steps, allergen controls).
Establish monitoring systems for key control points to ensure effective management and control.
Maintain records to prove compliance.
This proactive model empowers the FDA to prevent unsafe food from reaching consumers in the first place and to respond more quickly if a recall is necessary.
Key Provisions and Requirements Under FSMA
Rather than being a single regulation, FSMA is a framework comprising key rules, each covering different types of risks, processes, or parts of the supply chain.

Here’s a quick breakdown:
Rule | Scope | Main Requirements |
Preventive Controls (Human) | Processors & manufacturers | Food safety plan, hazard analysis, monitoring, verification |
Preventive Controls (Animal) | Feed mills, pet food makers | Hazard controls, cGMPs, and a written plan |
Produce Safety | Farms | Water testing, hygiene, composting, and equipment sanitation |
Sanitary Transportation | Truck/rail shipping | Equipment cleaning, temperature logs, training, recordkeeping |
Intentional Adulteration | High-risk facilities | Vulnerability analysis, mitigation strategies, and defense plans |
Foreign Supplier Verification | U.S. food importers | Risk-based supplier checks, documentation, and equal health protection |
Third-Party Certification | Importers (voluntary) | Accredited audits, faster clearance through VQIP |
Traceability Rule | High-risk foods | KDEs, CTEs, lot coding, fast-response records for outbreak tracking |
1. Preventive Controls for Human Food
The Preventive Controls for Human Food rule (21 CFR Part 117) applies to most facilities that manufacture, process, pack, or hold food.
The rule requires these businesses to implement a written food safety plan based on hazard analysis and risk-based preventive controls.
➸ What must facilities do?
Conduct a hazard analysis: Identify biological, chemical, and physical hazards for each product.
Implement preventive controls: Examples include pasteurization, sanitation procedures, allergen management, and metal detection.
Monitor controls: Utilize records such as temperature logs or test results.
Correct problems: Define actions for failed controls, such as discarding affected batches.
Verify the plan: Conduct product testing, environmental swabbing, and periodic reviews.
Assign a Qualified Individual (QI): Only someone with training or experience can prepare and oversee the plan.
All documentation must be kept on-site or be easily accessible during FDA inspections. This rule generalizes HACCP practices and emphasizes the importance of rigorous documentation.
Pro Tip
Signify automatically identifies conformity gaps by analyzing your documentation, processes, and products against regulatory requirements, helping you spot potential issues early.

With real-time insights, Signify provides specific remediation guidance to address non-conformities before they affect your certification status or market access.
2. Preventive Controls for Animal Food
Animal food producers, including those that manufacture pet food and livestock feed, are also subject to FSMA.
The Preventive Controls for Animal Food rule (21 CFR Part 507) mirrors the Preventive Controls for Human Food rule in structure.
➸ Core requirements include:
Following current Good Manufacturing Practices (cGMPs).
Conducting hazard analysis for risks like mycotoxins or Salmonella.
Implementing controls (e.g., pest control, supplier verification).
Maintaining a written feed safety plan.
Covered businesses range from feed mills to pet food processors. The goal is to stop contaminated animal food from harming pets, livestock, or the human food chain.
3. Produce Safety Standards
The Produce Safety Rule (21 CFR Part 112) establishes science-based standards for the growing, harvesting, packing, and holding of fruits and vegetables.
It was the first time U.S. federal law created mandatory on-farm food safety regulations.
➸ Focus areas:
Agricultural water: Test irrigation and wash water quality.
Worker hygiene: Provide handwashing stations and hygiene training.
Soil amendments: Apply compost safely.
Sanitation: Clean and maintain harvesting and packing equipment.
Animal intrusion: Prevent animal contamination of fields.
➸ Additional info:
Large farms (with sales exceeding $500,000) were required to comply by 2018; smaller farms followed through 2019–2020.
Very small farms (<$25,000 sales) are exempt.
By the end of 2025, all covered farms are expected to be compliant.
4. Sanitary Transportation Rule
The Sanitary Transportation of Human and Animal Food rule (21 CFR Part 1, Subpart O) applies to truck and rail shipments within the U.S. Its purpose is to prevent food from becoming unsafe during transit.
➸ Key requirements:
Clean equipment: Vehicles must be properly maintained and kept clean.
Temperature control: Cold foods must be maintained within safe temperature ranges.
Staff training: Workers must understand the importance of safe food handling.
Records: Keep documentation of sanitation, conditions, and corrective actions.
➸ Additional note: This rule does not cover air or marine shipments.
5. Intentional Adulteration (Food Defense)
The Mitigation Strategies to Protect Food Against Intentional Adulteration rule (21 CFR Part 121) requires large facilities to protect against deliberate attacks.
➸ Facilities must:
Identify vulnerable points for intentional contamination.
Implement mitigation strategies (e.g., restricted access, employee screening).
Monitor and verify those strategies.
Write a food defense plan.
This rule covers sabotage, terrorism, and insider threats, helping safeguard the food supply from rare but dangerous events.
6. Foreign Supplier Verification Program (FSVP)
Under 21 CFR Part 1 Subpart L, U.S. importers must ensure their foreign suppliers meet FDA standards.
➸ Responsibilities include:
Performing risk-based verification through audits, testing, or certifications.
Ensuring equal public health protection as U.S. counterparts.
Maintaining documentation showing due diligence.
This helps prevent unsafe imported foods from reaching U.S. consumers.
7. Third-Party Certification and VQIP
FSMA established a voluntary certification system for food imports.
➸ How it works:
The FDA accredits third-party auditors who certify foreign facilities.
Importers can join the Voluntary Qualified Importer Program (VQIP) for expedited clearance.
The FDA may require certification for certain high-risk imports.
This program adds accountability to global supply chains.
8. Traceability Requirements (FSMA Section 204)
Finalized in December 2022, the Food Traceability Rule enhances tracking for certain high-risk foods.
These include leafy greens, melons, nut butters, soft cheeses, shell eggs, and more.
➸ Businesses must:
Track Key Data Elements (KDEs) at Critical Tracking Events (CTEs), such as harvest, processing, or shipping.
Assign and maintain lot codes for traceability.
Provide records to the FDA within 24 hours during an investigation.

What’s New: FSMA 204(d) and Food Traceability
In 2025, one rule is garnering the most attention: Section 204(d), which introduces significant changes to food traceability.
➸ The goal? To make sure high-risk foods can be traced quickly in the event of contamination.
What Does FSMA 204(d) Require?
If you handle foods on the FDA’s Food Traceability List (FTL), you need to:
Track Critical Tracking Events (CTEs) like harvesting, receiving, transforming, and shipping.
Record Key Data Elements (KDEs) at each CTE. This includes origin, lot codes, dates, locations, and more.
Maintain traceability lot codes throughout the life of the product.
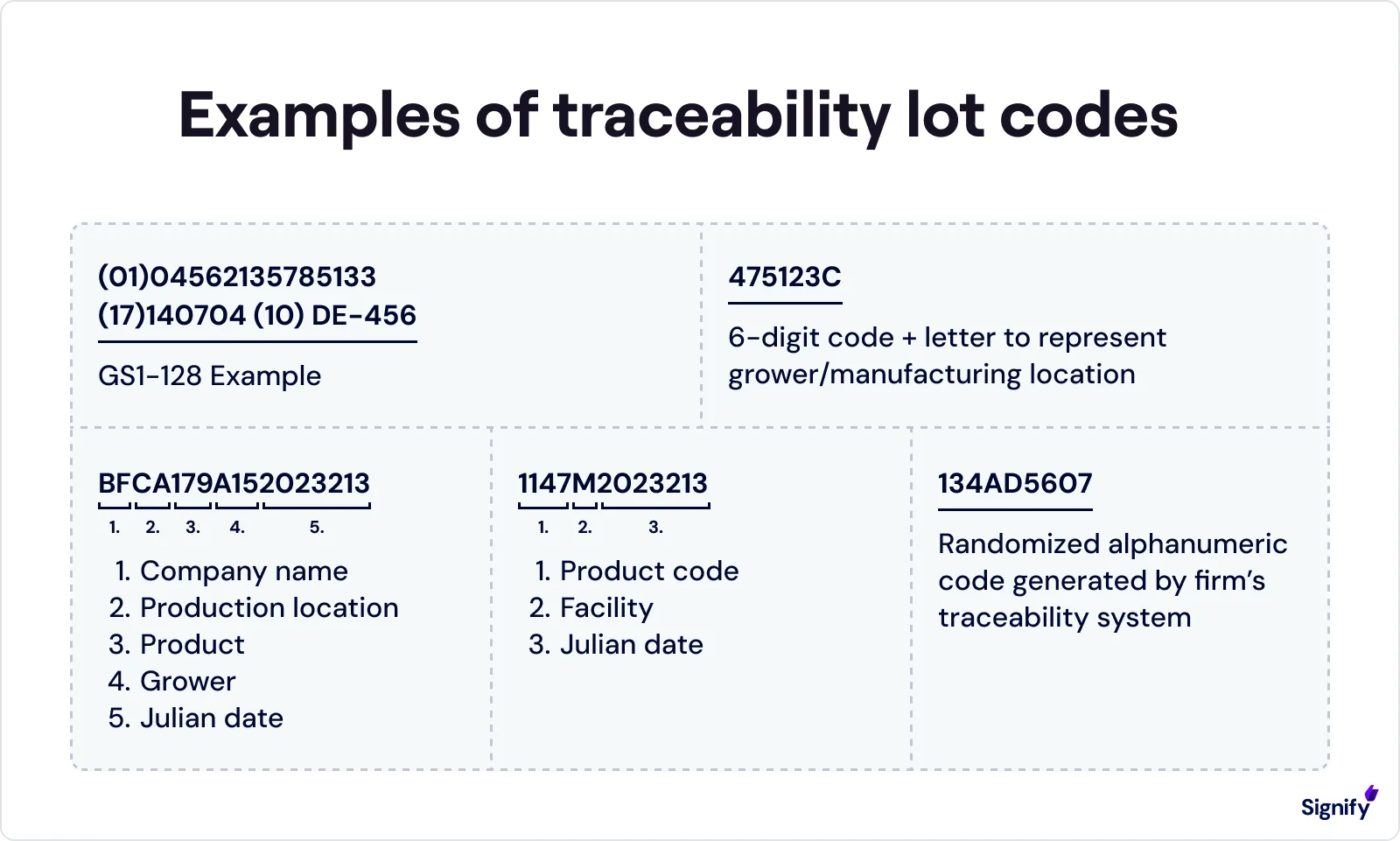
Respond to FDA requests for digital traceability records within 24 hours.
Which Foods Are on the FTL?
The list includes items with higher risk profiles. Some examples:
Leafy greens (e.g., romaine, spinach)
Fresh-cut fruits and vegetables
Soft cheeses
Shell eggs
Seafood like tuna and shrimp
Ready-to-eat deli salads
Regulatory Coverage and Compliance
FSMA covers a wide array of food-related businesses, both domestic and international, that fall under FDA jurisdiction.
This section outlines who is required to comply, how compliance is managed, and the enforcement mechanisms the FDA employs.
Who Is Covered by FSMA?
➸ Generally, FSMA applies to any facility that:
Manufactures, processes, packs, or holds food (human or animal).
Imports FDA-regulated food into the U.S.
Transports food (applies under the Sanitary Transportation rule).
➸ Covered entities include:
Food processors,
Farms (if they meet facility criteria),
Packing houses and warehouses,
Food transporters (truck or rail),
Importers of food.
➸ Exemptions and Special Cases:
USDA-regulated products, such as meat, poultry, and certain egg products, are not covered.
Very small farms (under ~$25,000/year in sales) are exempt from the Produce Safety Rule.
Qualified facilities (very small processors selling directly to consumers) may have scaled-down compliance duties.
Facility Registration and Inspections
Under FSMA, all covered facilities must:
Register with the FDA.
Renew registration every two years.
Registration ties the facility directly to FDA oversight.
If a facility’s food is linked to a serious health risk, the FDA has the authority to suspend its registration, which stops it from distributing food altogether.
➸ FDA Inspection Schedule:
High-risk facilities: Every 3 years
Lower-risk facilities: Every 5–7 years
For imported food, the FDA began inspecting foreign facilities in 2012 and increased inspections yearly.
Scaled Compliance for Small Businesses
Recognizing that one size doesn’t fit all, FSMA includes modified requirements for small and very small businesses.
These adjustments are especially relevant for small farms and producers with direct-to-consumer models.
➸ Examples of Scaled Compliance:
Preventive Controls Rule:
"Very small businesses" (<$1M in annual sales) follow simplified provisions
Exempt if primarily selling direct to consumers
Produce Safety Rule:
Fully exempt under $25,000 in average annual sales
Extended deadlines for mid-sized farms
The FDA also offers detailed flowcharts and compliance tools to help businesses determine their obligations.
Food Safety Plans and Documentation
A core principle of FSMA is documented accountability.
Covered businesses must maintain detailed records to demonstrate they are meeting food safety obligations.
➸ Required Documentation:
Preventive Controls facilities: Written food safety plans, hazard analyses, training logs, test results, and corrective actions.
Produce farms: Farm-specific food safety plans, including water testing results and sanitation protocols.
Traceability-covered businesses: KDEs, CTEs, and lot tracking per FSMA Section 204
These records must be readily accessible and produced upon request by the FDA during inspections.
FDA Inspections and Enforcement Tools
FSMA gives the FDA broad authority to enforce compliance.
Inspectors can:
Review all required records on-site.
Conduct unannounced inspections.
Detain shipments suspected of contamination.
Order mandatory recalls if a company fails to act.
Suspend facility registration if food poses imminent health hazards.
The FDA also maintains a public dashboard that tracks FSMA inspection and enforcement metrics, providing transparency into the agency's actions.
FSMA Developments and Future Outlook
FSMA continues to evolve as the FDA adjusts rules and responds to industry needs.
Below is a breakdown of key recent updates and what to expect next:
Agricultural Water Testing Tightened (2024): The FDA finalized stricter requirements for pre-harvest agricultural water testing on produce farms to reduce microbial contamination risks, particularly for leafy greens and other fresh produce.
FSMA Traceability Rule Refinements: Although the Food Traceability Rule officially took effect, the FDA proposed exemptions for certain low-risk foods, such as cottage cheese (2024), indicating its intention to prioritize higher-risk categories.
Sprout Safety Rule and Produce Guidance: New rules and guidance issued between 2023 and 2024 include a finalized Sprout Safety Rule targeting high-risk sprouted seeds and updated produce safety guidance aligning with FSMA standards.
Training and Technical Assistance Programs Expanded: The FDA continues to support compliance through resources such as Produce Safety Alliance materials, PCQI (Preventive Controls Qualified Individual) training, and upcoming 2025 draft guidance on sanitation for low-moisture ready-to-eat foods (e.g., spices, powdered products).
“New Era of Smarter Food Safety” (2020–ongoing): Although not a legal part of FSMA, the FDA’s initiative promotes the use of AI, blockchain, and sensors to enhance traceability and predictive risk assessment, aligning with FSMA’s preventive goals.
How Signify Supports FSMA Compliance in Food Manufacturing
Signify is an AI compliance agent and an all-in-one platform for food manufacturers.
It helps you meet FSMA traceability and documentation requirements by automating labeling checks, monitoring regulatory updates, and managing traceability records.
Our key features include:
Proactive Compliance Monitoring: Scans labeling and traceability data to flag issues early, reducing non-compliance risk.Smart Documentation Management: Organizes all your KDEs and CTEs in one place for fast access during audits or recalls.
Real-Time Regulatory Updates: Tracks FDA rule changes and alerts you to anything that could impact your compliance.
Guided Remediation: Provides step-by-step actions to address gaps in traceability or labeling, aligning with FSMA standards.
Built-In AI Compliance Agents: Specialized for consumer goods, they continuously monitor your workflows and documentation.
Try it now for free and see how Signify keeps your food manufacturing operations audit-ready and compliant with FSMA.
The FDA’s enforcement of the Food Safety Modernization Act (FSMA) is designed to strengthen public health protections, prevent foodborne illness, and ensure traceability across the supply chain.
In this guide, we’ll break down the core Food Safety Modernization Act compliance areas, walk through key regulatory checks, and share practical tips for food businesses to meet FDA standards and avoid costly disruptions.
Let’s get started.
What is FSMA?
The Food Safety Modernization Act (FSMA) is a landmark U.S. law, signed by President Obama on January 4, 2011, aimed at fundamentally transforming the nation’s food safety system.
FSMA shifted the model from reactive (i.e., responding to outbreaks) to proactive (i.e., preventing them).
It gave the FDA sweeping new authorities, including mandatory recalls, risk-based standards, farm-to-fork traceability, and enhanced global oversight, enabling food safety grounded in science and prevention.
➸ At a Glance: Key Facts
Signing date: January 4, 2011
Primary aim: Prevention-first food safety coordinated across the entire food chain
Health imperative: Based on CDC estimates (~48 million illnesses, ~3,000 deaths per year)

FSMA's scope encompasses farms, manufacturers, transporters, and importers, requiring written preventive plans, stronger inspections, and thorough recordkeeping.
FSMA’s Core Goal: Prevention Over Reaction
Instead of waiting for contamination to cause outbreaks, FSMA requires businesses to:
Identify hazards early in the supply chain to prevent potential issues.
Implement preventive controls (e.g., kill steps, allergen controls).
Establish monitoring systems for key control points to ensure effective management and control.
Maintain records to prove compliance.
This proactive model empowers the FDA to prevent unsafe food from reaching consumers in the first place and to respond more quickly if a recall is necessary.
Key Provisions and Requirements Under FSMA
Rather than being a single regulation, FSMA is a framework comprising key rules, each covering different types of risks, processes, or parts of the supply chain.

Here’s a quick breakdown:
Rule | Scope | Main Requirements |
Preventive Controls (Human) | Processors & manufacturers | Food safety plan, hazard analysis, monitoring, verification |
Preventive Controls (Animal) | Feed mills, pet food makers | Hazard controls, cGMPs, and a written plan |
Produce Safety | Farms | Water testing, hygiene, composting, and equipment sanitation |
Sanitary Transportation | Truck/rail shipping | Equipment cleaning, temperature logs, training, recordkeeping |
Intentional Adulteration | High-risk facilities | Vulnerability analysis, mitigation strategies, and defense plans |
Foreign Supplier Verification | U.S. food importers | Risk-based supplier checks, documentation, and equal health protection |
Third-Party Certification | Importers (voluntary) | Accredited audits, faster clearance through VQIP |
Traceability Rule | High-risk foods | KDEs, CTEs, lot coding, fast-response records for outbreak tracking |
1. Preventive Controls for Human Food
The Preventive Controls for Human Food rule (21 CFR Part 117) applies to most facilities that manufacture, process, pack, or hold food.
The rule requires these businesses to implement a written food safety plan based on hazard analysis and risk-based preventive controls.
➸ What must facilities do?
Conduct a hazard analysis: Identify biological, chemical, and physical hazards for each product.
Implement preventive controls: Examples include pasteurization, sanitation procedures, allergen management, and metal detection.
Monitor controls: Utilize records such as temperature logs or test results.
Correct problems: Define actions for failed controls, such as discarding affected batches.
Verify the plan: Conduct product testing, environmental swabbing, and periodic reviews.
Assign a Qualified Individual (QI): Only someone with training or experience can prepare and oversee the plan.
All documentation must be kept on-site or be easily accessible during FDA inspections. This rule generalizes HACCP practices and emphasizes the importance of rigorous documentation.
Pro Tip
Signify automatically identifies conformity gaps by analyzing your documentation, processes, and products against regulatory requirements, helping you spot potential issues early.

With real-time insights, Signify provides specific remediation guidance to address non-conformities before they affect your certification status or market access.
2. Preventive Controls for Animal Food
Animal food producers, including those that manufacture pet food and livestock feed, are also subject to FSMA.
The Preventive Controls for Animal Food rule (21 CFR Part 507) mirrors the Preventive Controls for Human Food rule in structure.
➸ Core requirements include:
Following current Good Manufacturing Practices (cGMPs).
Conducting hazard analysis for risks like mycotoxins or Salmonella.
Implementing controls (e.g., pest control, supplier verification).
Maintaining a written feed safety plan.
Covered businesses range from feed mills to pet food processors. The goal is to stop contaminated animal food from harming pets, livestock, or the human food chain.
3. Produce Safety Standards
The Produce Safety Rule (21 CFR Part 112) establishes science-based standards for the growing, harvesting, packing, and holding of fruits and vegetables.
It was the first time U.S. federal law created mandatory on-farm food safety regulations.
➸ Focus areas:
Agricultural water: Test irrigation and wash water quality.
Worker hygiene: Provide handwashing stations and hygiene training.
Soil amendments: Apply compost safely.
Sanitation: Clean and maintain harvesting and packing equipment.
Animal intrusion: Prevent animal contamination of fields.
➸ Additional info:
Large farms (with sales exceeding $500,000) were required to comply by 2018; smaller farms followed through 2019–2020.
Very small farms (<$25,000 sales) are exempt.
By the end of 2025, all covered farms are expected to be compliant.
4. Sanitary Transportation Rule
The Sanitary Transportation of Human and Animal Food rule (21 CFR Part 1, Subpart O) applies to truck and rail shipments within the U.S. Its purpose is to prevent food from becoming unsafe during transit.
➸ Key requirements:
Clean equipment: Vehicles must be properly maintained and kept clean.
Temperature control: Cold foods must be maintained within safe temperature ranges.
Staff training: Workers must understand the importance of safe food handling.
Records: Keep documentation of sanitation, conditions, and corrective actions.
➸ Additional note: This rule does not cover air or marine shipments.
5. Intentional Adulteration (Food Defense)
The Mitigation Strategies to Protect Food Against Intentional Adulteration rule (21 CFR Part 121) requires large facilities to protect against deliberate attacks.
➸ Facilities must:
Identify vulnerable points for intentional contamination.
Implement mitigation strategies (e.g., restricted access, employee screening).
Monitor and verify those strategies.
Write a food defense plan.
This rule covers sabotage, terrorism, and insider threats, helping safeguard the food supply from rare but dangerous events.
6. Foreign Supplier Verification Program (FSVP)
Under 21 CFR Part 1 Subpart L, U.S. importers must ensure their foreign suppliers meet FDA standards.
➸ Responsibilities include:
Performing risk-based verification through audits, testing, or certifications.
Ensuring equal public health protection as U.S. counterparts.
Maintaining documentation showing due diligence.
This helps prevent unsafe imported foods from reaching U.S. consumers.
7. Third-Party Certification and VQIP
FSMA established a voluntary certification system for food imports.
➸ How it works:
The FDA accredits third-party auditors who certify foreign facilities.
Importers can join the Voluntary Qualified Importer Program (VQIP) for expedited clearance.
The FDA may require certification for certain high-risk imports.
This program adds accountability to global supply chains.
8. Traceability Requirements (FSMA Section 204)
Finalized in December 2022, the Food Traceability Rule enhances tracking for certain high-risk foods.
These include leafy greens, melons, nut butters, soft cheeses, shell eggs, and more.
➸ Businesses must:
Track Key Data Elements (KDEs) at Critical Tracking Events (CTEs), such as harvest, processing, or shipping.
Assign and maintain lot codes for traceability.
Provide records to the FDA within 24 hours during an investigation.

What’s New: FSMA 204(d) and Food Traceability
In 2025, one rule is garnering the most attention: Section 204(d), which introduces significant changes to food traceability.
➸ The goal? To make sure high-risk foods can be traced quickly in the event of contamination.
What Does FSMA 204(d) Require?
If you handle foods on the FDA’s Food Traceability List (FTL), you need to:
Track Critical Tracking Events (CTEs) like harvesting, receiving, transforming, and shipping.
Record Key Data Elements (KDEs) at each CTE. This includes origin, lot codes, dates, locations, and more.
Maintain traceability lot codes throughout the life of the product.

Respond to FDA requests for digital traceability records within 24 hours.
Which Foods Are on the FTL?
The list includes items with higher risk profiles. Some examples:
Leafy greens (e.g., romaine, spinach)
Fresh-cut fruits and vegetables
Soft cheeses
Shell eggs
Seafood like tuna and shrimp
Ready-to-eat deli salads
Regulatory Coverage and Compliance
FSMA covers a wide array of food-related businesses, both domestic and international, that fall under FDA jurisdiction.
This section outlines who is required to comply, how compliance is managed, and the enforcement mechanisms the FDA employs.
Who Is Covered by FSMA?
➸ Generally, FSMA applies to any facility that:
Manufactures, processes, packs, or holds food (human or animal).
Imports FDA-regulated food into the U.S.
Transports food (applies under the Sanitary Transportation rule).
➸ Covered entities include:
Food processors,
Farms (if they meet facility criteria),
Packing houses and warehouses,
Food transporters (truck or rail),
Importers of food.
➸ Exemptions and Special Cases:
USDA-regulated products, such as meat, poultry, and certain egg products, are not covered.
Very small farms (under ~$25,000/year in sales) are exempt from the Produce Safety Rule.
Qualified facilities (very small processors selling directly to consumers) may have scaled-down compliance duties.
Facility Registration and Inspections
Under FSMA, all covered facilities must:
Register with the FDA.
Renew registration every two years.
Registration ties the facility directly to FDA oversight.
If a facility’s food is linked to a serious health risk, the FDA has the authority to suspend its registration, which stops it from distributing food altogether.
➸ FDA Inspection Schedule:
High-risk facilities: Every 3 years
Lower-risk facilities: Every 5–7 years
For imported food, the FDA began inspecting foreign facilities in 2012 and increased inspections yearly.
Scaled Compliance for Small Businesses
Recognizing that one size doesn’t fit all, FSMA includes modified requirements for small and very small businesses.
These adjustments are especially relevant for small farms and producers with direct-to-consumer models.
➸ Examples of Scaled Compliance:
Preventive Controls Rule:
"Very small businesses" (<$1M in annual sales) follow simplified provisions
Exempt if primarily selling direct to consumers
Produce Safety Rule:
Fully exempt under $25,000 in average annual sales
Extended deadlines for mid-sized farms
The FDA also offers detailed flowcharts and compliance tools to help businesses determine their obligations.
Food Safety Plans and Documentation
A core principle of FSMA is documented accountability.
Covered businesses must maintain detailed records to demonstrate they are meeting food safety obligations.
➸ Required Documentation:
Preventive Controls facilities: Written food safety plans, hazard analyses, training logs, test results, and corrective actions.
Produce farms: Farm-specific food safety plans, including water testing results and sanitation protocols.
Traceability-covered businesses: KDEs, CTEs, and lot tracking per FSMA Section 204
These records must be readily accessible and produced upon request by the FDA during inspections.
FDA Inspections and Enforcement Tools
FSMA gives the FDA broad authority to enforce compliance.
Inspectors can:
Review all required records on-site.
Conduct unannounced inspections.
Detain shipments suspected of contamination.
Order mandatory recalls if a company fails to act.
Suspend facility registration if food poses imminent health hazards.
The FDA also maintains a public dashboard that tracks FSMA inspection and enforcement metrics, providing transparency into the agency's actions.
FSMA Developments and Future Outlook
FSMA continues to evolve as the FDA adjusts rules and responds to industry needs.
Below is a breakdown of key recent updates and what to expect next:
Agricultural Water Testing Tightened (2024): The FDA finalized stricter requirements for pre-harvest agricultural water testing on produce farms to reduce microbial contamination risks, particularly for leafy greens and other fresh produce.
FSMA Traceability Rule Refinements: Although the Food Traceability Rule officially took effect, the FDA proposed exemptions for certain low-risk foods, such as cottage cheese (2024), indicating its intention to prioritize higher-risk categories.
Sprout Safety Rule and Produce Guidance: New rules and guidance issued between 2023 and 2024 include a finalized Sprout Safety Rule targeting high-risk sprouted seeds and updated produce safety guidance aligning with FSMA standards.
Training and Technical Assistance Programs Expanded: The FDA continues to support compliance through resources such as Produce Safety Alliance materials, PCQI (Preventive Controls Qualified Individual) training, and upcoming 2025 draft guidance on sanitation for low-moisture ready-to-eat foods (e.g., spices, powdered products).
“New Era of Smarter Food Safety” (2020–ongoing): Although not a legal part of FSMA, the FDA’s initiative promotes the use of AI, blockchain, and sensors to enhance traceability and predictive risk assessment, aligning with FSMA’s preventive goals.
How Signify Supports FSMA Compliance in Food Manufacturing
Signify is an AI compliance agent and an all-in-one platform for food manufacturers.
It helps you meet FSMA traceability and documentation requirements by automating labeling checks, monitoring regulatory updates, and managing traceability records.
Our key features include:
Proactive Compliance Monitoring: Scans labeling and traceability data to flag issues early, reducing non-compliance risk.Smart Documentation Management: Organizes all your KDEs and CTEs in one place for fast access during audits or recalls.
Real-Time Regulatory Updates: Tracks FDA rule changes and alerts you to anything that could impact your compliance.
Guided Remediation: Provides step-by-step actions to address gaps in traceability or labeling, aligning with FSMA standards.
Built-In AI Compliance Agents: Specialized for consumer goods, they continuously monitor your workflows and documentation.
Try it now for free and see how Signify keeps your food manufacturing operations audit-ready and compliant with FSMA.
The FDA’s enforcement of the Food Safety Modernization Act (FSMA) is designed to strengthen public health protections, prevent foodborne illness, and ensure traceability across the supply chain.
In this guide, we’ll break down the core Food Safety Modernization Act compliance areas, walk through key regulatory checks, and share practical tips for food businesses to meet FDA standards and avoid costly disruptions.
Let’s get started.
What is FSMA?
The Food Safety Modernization Act (FSMA) is a landmark U.S. law, signed by President Obama on January 4, 2011, aimed at fundamentally transforming the nation’s food safety system.
FSMA shifted the model from reactive (i.e., responding to outbreaks) to proactive (i.e., preventing them).
It gave the FDA sweeping new authorities, including mandatory recalls, risk-based standards, farm-to-fork traceability, and enhanced global oversight, enabling food safety grounded in science and prevention.
➸ At a Glance: Key Facts
Signing date: January 4, 2011
Primary aim: Prevention-first food safety coordinated across the entire food chain
Health imperative: Based on CDC estimates (~48 million illnesses, ~3,000 deaths per year)

FSMA's scope encompasses farms, manufacturers, transporters, and importers, requiring written preventive plans, stronger inspections, and thorough recordkeeping.
FSMA’s Core Goal: Prevention Over Reaction
Instead of waiting for contamination to cause outbreaks, FSMA requires businesses to:
Identify hazards early in the supply chain to prevent potential issues.
Implement preventive controls (e.g., kill steps, allergen controls).
Establish monitoring systems for key control points to ensure effective management and control.
Maintain records to prove compliance.
This proactive model empowers the FDA to prevent unsafe food from reaching consumers in the first place and to respond more quickly if a recall is necessary.
Key Provisions and Requirements Under FSMA
Rather than being a single regulation, FSMA is a framework comprising key rules, each covering different types of risks, processes, or parts of the supply chain.

Here’s a quick breakdown:
Rule | Scope | Main Requirements |
Preventive Controls (Human) | Processors & manufacturers | Food safety plan, hazard analysis, monitoring, verification |
Preventive Controls (Animal) | Feed mills, pet food makers | Hazard controls, cGMPs, and a written plan |
Produce Safety | Farms | Water testing, hygiene, composting, and equipment sanitation |
Sanitary Transportation | Truck/rail shipping | Equipment cleaning, temperature logs, training, recordkeeping |
Intentional Adulteration | High-risk facilities | Vulnerability analysis, mitigation strategies, and defense plans |
Foreign Supplier Verification | U.S. food importers | Risk-based supplier checks, documentation, and equal health protection |
Third-Party Certification | Importers (voluntary) | Accredited audits, faster clearance through VQIP |
Traceability Rule | High-risk foods | KDEs, CTEs, lot coding, fast-response records for outbreak tracking |
1. Preventive Controls for Human Food
The Preventive Controls for Human Food rule (21 CFR Part 117) applies to most facilities that manufacture, process, pack, or hold food.
The rule requires these businesses to implement a written food safety plan based on hazard analysis and risk-based preventive controls.
➸ What must facilities do?
Conduct a hazard analysis: Identify biological, chemical, and physical hazards for each product.
Implement preventive controls: Examples include pasteurization, sanitation procedures, allergen management, and metal detection.
Monitor controls: Utilize records such as temperature logs or test results.
Correct problems: Define actions for failed controls, such as discarding affected batches.
Verify the plan: Conduct product testing, environmental swabbing, and periodic reviews.
Assign a Qualified Individual (QI): Only someone with training or experience can prepare and oversee the plan.
All documentation must be kept on-site or be easily accessible during FDA inspections. This rule generalizes HACCP practices and emphasizes the importance of rigorous documentation.
Pro Tip
Signify automatically identifies conformity gaps by analyzing your documentation, processes, and products against regulatory requirements, helping you spot potential issues early.

With real-time insights, Signify provides specific remediation guidance to address non-conformities before they affect your certification status or market access.
2. Preventive Controls for Animal Food
Animal food producers, including those that manufacture pet food and livestock feed, are also subject to FSMA.
The Preventive Controls for Animal Food rule (21 CFR Part 507) mirrors the Preventive Controls for Human Food rule in structure.
➸ Core requirements include:
Following current Good Manufacturing Practices (cGMPs).
Conducting hazard analysis for risks like mycotoxins or Salmonella.
Implementing controls (e.g., pest control, supplier verification).
Maintaining a written feed safety plan.
Covered businesses range from feed mills to pet food processors. The goal is to stop contaminated animal food from harming pets, livestock, or the human food chain.
3. Produce Safety Standards
The Produce Safety Rule (21 CFR Part 112) establishes science-based standards for the growing, harvesting, packing, and holding of fruits and vegetables.
It was the first time U.S. federal law created mandatory on-farm food safety regulations.
➸ Focus areas:
Agricultural water: Test irrigation and wash water quality.
Worker hygiene: Provide handwashing stations and hygiene training.
Soil amendments: Apply compost safely.
Sanitation: Clean and maintain harvesting and packing equipment.
Animal intrusion: Prevent animal contamination of fields.
➸ Additional info:
Large farms (with sales exceeding $500,000) were required to comply by 2018; smaller farms followed through 2019–2020.
Very small farms (<$25,000 sales) are exempt.
By the end of 2025, all covered farms are expected to be compliant.
4. Sanitary Transportation Rule
The Sanitary Transportation of Human and Animal Food rule (21 CFR Part 1, Subpart O) applies to truck and rail shipments within the U.S. Its purpose is to prevent food from becoming unsafe during transit.
➸ Key requirements:
Clean equipment: Vehicles must be properly maintained and kept clean.
Temperature control: Cold foods must be maintained within safe temperature ranges.
Staff training: Workers must understand the importance of safe food handling.
Records: Keep documentation of sanitation, conditions, and corrective actions.
➸ Additional note: This rule does not cover air or marine shipments.
5. Intentional Adulteration (Food Defense)
The Mitigation Strategies to Protect Food Against Intentional Adulteration rule (21 CFR Part 121) requires large facilities to protect against deliberate attacks.
➸ Facilities must:
Identify vulnerable points for intentional contamination.
Implement mitigation strategies (e.g., restricted access, employee screening).
Monitor and verify those strategies.
Write a food defense plan.
This rule covers sabotage, terrorism, and insider threats, helping safeguard the food supply from rare but dangerous events.
6. Foreign Supplier Verification Program (FSVP)
Under 21 CFR Part 1 Subpart L, U.S. importers must ensure their foreign suppliers meet FDA standards.
➸ Responsibilities include:
Performing risk-based verification through audits, testing, or certifications.
Ensuring equal public health protection as U.S. counterparts.
Maintaining documentation showing due diligence.
This helps prevent unsafe imported foods from reaching U.S. consumers.
7. Third-Party Certification and VQIP
FSMA established a voluntary certification system for food imports.
➸ How it works:
The FDA accredits third-party auditors who certify foreign facilities.
Importers can join the Voluntary Qualified Importer Program (VQIP) for expedited clearance.
The FDA may require certification for certain high-risk imports.
This program adds accountability to global supply chains.
8. Traceability Requirements (FSMA Section 204)
Finalized in December 2022, the Food Traceability Rule enhances tracking for certain high-risk foods.
These include leafy greens, melons, nut butters, soft cheeses, shell eggs, and more.
➸ Businesses must:
Track Key Data Elements (KDEs) at Critical Tracking Events (CTEs), such as harvest, processing, or shipping.
Assign and maintain lot codes for traceability.
Provide records to the FDA within 24 hours during an investigation.

What’s New: FSMA 204(d) and Food Traceability
In 2025, one rule is garnering the most attention: Section 204(d), which introduces significant changes to food traceability.
➸ The goal? To make sure high-risk foods can be traced quickly in the event of contamination.
What Does FSMA 204(d) Require?
If you handle foods on the FDA’s Food Traceability List (FTL), you need to:
Track Critical Tracking Events (CTEs) like harvesting, receiving, transforming, and shipping.
Record Key Data Elements (KDEs) at each CTE. This includes origin, lot codes, dates, locations, and more.
Maintain traceability lot codes throughout the life of the product.

Respond to FDA requests for digital traceability records within 24 hours.
Which Foods Are on the FTL?
The list includes items with higher risk profiles. Some examples:
Leafy greens (e.g., romaine, spinach)
Fresh-cut fruits and vegetables
Soft cheeses
Shell eggs
Seafood like tuna and shrimp
Ready-to-eat deli salads
Regulatory Coverage and Compliance
FSMA covers a wide array of food-related businesses, both domestic and international, that fall under FDA jurisdiction.
This section outlines who is required to comply, how compliance is managed, and the enforcement mechanisms the FDA employs.
Who Is Covered by FSMA?
➸ Generally, FSMA applies to any facility that:
Manufactures, processes, packs, or holds food (human or animal).
Imports FDA-regulated food into the U.S.
Transports food (applies under the Sanitary Transportation rule).
➸ Covered entities include:
Food processors,
Farms (if they meet facility criteria),
Packing houses and warehouses,
Food transporters (truck or rail),
Importers of food.
➸ Exemptions and Special Cases:
USDA-regulated products, such as meat, poultry, and certain egg products, are not covered.
Very small farms (under ~$25,000/year in sales) are exempt from the Produce Safety Rule.
Qualified facilities (very small processors selling directly to consumers) may have scaled-down compliance duties.
Facility Registration and Inspections
Under FSMA, all covered facilities must:
Register with the FDA.
Renew registration every two years.
Registration ties the facility directly to FDA oversight.
If a facility’s food is linked to a serious health risk, the FDA has the authority to suspend its registration, which stops it from distributing food altogether.
➸ FDA Inspection Schedule:
High-risk facilities: Every 3 years
Lower-risk facilities: Every 5–7 years
For imported food, the FDA began inspecting foreign facilities in 2012 and increased inspections yearly.
Scaled Compliance for Small Businesses
Recognizing that one size doesn’t fit all, FSMA includes modified requirements for small and very small businesses.
These adjustments are especially relevant for small farms and producers with direct-to-consumer models.
➸ Examples of Scaled Compliance:
Preventive Controls Rule:
"Very small businesses" (<$1M in annual sales) follow simplified provisions
Exempt if primarily selling direct to consumers
Produce Safety Rule:
Fully exempt under $25,000 in average annual sales
Extended deadlines for mid-sized farms
The FDA also offers detailed flowcharts and compliance tools to help businesses determine their obligations.
Food Safety Plans and Documentation
A core principle of FSMA is documented accountability.
Covered businesses must maintain detailed records to demonstrate they are meeting food safety obligations.
➸ Required Documentation:
Preventive Controls facilities: Written food safety plans, hazard analyses, training logs, test results, and corrective actions.
Produce farms: Farm-specific food safety plans, including water testing results and sanitation protocols.
Traceability-covered businesses: KDEs, CTEs, and lot tracking per FSMA Section 204
These records must be readily accessible and produced upon request by the FDA during inspections.
FDA Inspections and Enforcement Tools
FSMA gives the FDA broad authority to enforce compliance.
Inspectors can:
Review all required records on-site.
Conduct unannounced inspections.
Detain shipments suspected of contamination.
Order mandatory recalls if a company fails to act.
Suspend facility registration if food poses imminent health hazards.
The FDA also maintains a public dashboard that tracks FSMA inspection and enforcement metrics, providing transparency into the agency's actions.
FSMA Developments and Future Outlook
FSMA continues to evolve as the FDA adjusts rules and responds to industry needs.
Below is a breakdown of key recent updates and what to expect next:
Agricultural Water Testing Tightened (2024): The FDA finalized stricter requirements for pre-harvest agricultural water testing on produce farms to reduce microbial contamination risks, particularly for leafy greens and other fresh produce.
FSMA Traceability Rule Refinements: Although the Food Traceability Rule officially took effect, the FDA proposed exemptions for certain low-risk foods, such as cottage cheese (2024), indicating its intention to prioritize higher-risk categories.
Sprout Safety Rule and Produce Guidance: New rules and guidance issued between 2023 and 2024 include a finalized Sprout Safety Rule targeting high-risk sprouted seeds and updated produce safety guidance aligning with FSMA standards.
Training and Technical Assistance Programs Expanded: The FDA continues to support compliance through resources such as Produce Safety Alliance materials, PCQI (Preventive Controls Qualified Individual) training, and upcoming 2025 draft guidance on sanitation for low-moisture ready-to-eat foods (e.g., spices, powdered products).
“New Era of Smarter Food Safety” (2020–ongoing): Although not a legal part of FSMA, the FDA’s initiative promotes the use of AI, blockchain, and sensors to enhance traceability and predictive risk assessment, aligning with FSMA’s preventive goals.
How Signify Supports FSMA Compliance in Food Manufacturing
Signify is an AI compliance agent and an all-in-one platform for food manufacturers.
It helps you meet FSMA traceability and documentation requirements by automating labeling checks, monitoring regulatory updates, and managing traceability records.
Our key features include:
Proactive Compliance Monitoring: Scans labeling and traceability data to flag issues early, reducing non-compliance risk.Smart Documentation Management: Organizes all your KDEs and CTEs in one place for fast access during audits or recalls.
Real-Time Regulatory Updates: Tracks FDA rule changes and alerts you to anything that could impact your compliance.
Guided Remediation: Provides step-by-step actions to address gaps in traceability or labeling, aligning with FSMA standards.
Built-In AI Compliance Agents: Specialized for consumer goods, they continuously monitor your workflows and documentation.
Try it now for free and see how Signify keeps your food manufacturing operations audit-ready and compliant with FSMA.
The information presented is for educational and informational purposes only and should not be construed as legal, regulatory, or professional advice. Organizations should consult with qualified legal and compliance professionals for guidance specific to their circumstances.
What is the Food Safety Modernization Act (FSMA)? [Guide]
What is the Food Safety Modernization Act (FSMA)? [Guide]
Jun 18, 2025